Genexine unveiled its plans to launch its Covid-19 vaccine within the year during the 2021 J.P. Morgan Healthcare Conference.
“Multinational pharmaceutical companies, such as Modena and Pfizer, have succeeded in developing vaccines. As far as Korea is concerned, however, Genexine is the front-runner in vaccine development,” Genexine Vice President Woo Jung-won said.
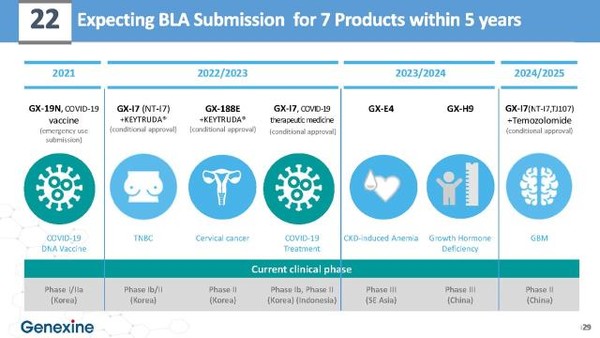
Woo noted that Genexine is undergoing phase 1 and 2 clinical trials for GX-19N, its Covid-19 vaccine candidate, after receiving approval from the Ministry of Food and Drug Safety in December.
The company initially obtained phase 1 and 2a clinical approval from the drug regulator for GX-19 in June last year, but had changed the substance to GX-19N after undergoing supplementation, she added. Despite the change in candidate substance, Woo said, Genexine plans to go along with its original schedule of launching the vaccine in 2021.
“We have proved the vaccine’s efficacy scientifically through animal experiments,” Woo said. “As the demand for the vaccine is rising, we will shorten the clinical period by using the emergency use approval (EUA) system.”
Woo also explained the company’s goals to apply for conditional approval for GX-188E, its cervical cancer vaccine, within this year.
“GX-188E is a therapeutic gene vaccine that combines DNA vector technology and immunity enhancement technology,” Woo said. “It works by putting DNA genetic information equivalent to E6, E7 protein of human papillomavirus (HPV) type 16 or 18 types into a plasmid vector and inject it into the human body.”
After the plasmid vector infiltrates into cells, it brings out HPV antigen, inducing an antigen-specific immune response and producing therapeutic effects, Woo added.
Woo noted that the vaccine had secured positive results as a combination treatment with Keytruda in a global phase 2 clinical trial that evaluated its efficacy. The drug regulator selected the vaccine as part of its rapid commercialization support project.
“The company has accumulated business capabilities through open innovation over the years, and we will increase corporate value by commercializing a total of seven pipelines within five years,” Woo said. “By 2030, we plan to launch 15 new drugs in various fields, including anticancer vaccines and gene therapy products.”

