U.S. researchers said they have developed a blood test that can detect over 50 types of cancer simultaneously.
The test showed higher sensitivity in patients with more disease progression, and it detected cancer in nine out of 10 stage-4 cancer patients.
The research team, led by Dr. Eric A. Klein at the Glickman Urological and Kidney Institute of Cleveland Clinic in Cleveland, published the study results that evaluated the effect of the multi-cancer early detection (MCED) test in the Annals of Oncology on Thursday.
The MCED test finds whether cell-free DNA (cfDNA) exists in cancer cells in the blood.
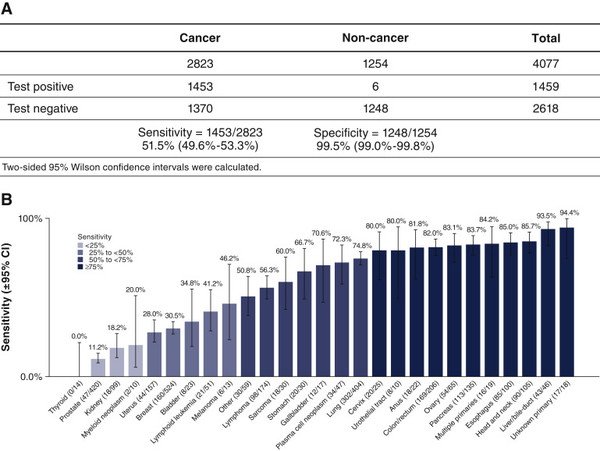
The study measured the MCED test’s specificity, sensitivity, and cancer signal origin (CSO) in 4,077 participants – 2,823 cancer patients and 1,254 without cancer.
The researchers measured sensitivity with the proportion of participants with a positive test result among cancer patients and specificity with the proportion of people with a negative test result among non-cancer participants.
The results showed that overall sensitivity for cancer signal detection was 51.5 percent, and sensitivity increased with the cancer stage.
Sensitivity among patients with stage 1 cancer was 16.8 percent, but it went up to 40.4 percent among stage 2 patients, 77 percent among stage 3 patients, and 90.1 percent among stage 4 patients.
Stage 1-3 sensitivity was 67.6 percent in 12 pre-specified cancers that account for approximately two-thirds of annual cancer deaths in the U.S., the research team said. The 12 cancer classes are the anus, bladder, colon/rectum, esophagus, head and neck, liver/bile duct, lung, lymphoma, ovary, pancreas, plasma cell neoplasm, and stomach.
The test detected CSO in more than 50 types of cancer, and the sensitivity was 40.7 percent in all cancer types.
The MCED test showed higher sensitivity in cancers that were difficult to detect than those with effective screening methods.
The test’s sensitivity in all-stage breast, colorectal, cervical, and prostate cancer was 33.7 percent. However, common screening options were already available for these cancers in the U.S., the research team said.
The sensitivity for tumors such as the esophagus, liver, and pancreatic cancer, which were difficult to detect early, was 65.6 percent twice that for cancers with common screening tools, the study added.
Specificity was 99.5 percent, meaning that a false-positive rate was only 0.5 percent. CSO accuracy in true positives was 88.7 percent, meaning that the test detected cancer issues accurately in 88.7 percent of cancer patients.
The research team concluded that the MCED test showed high specificity and accuracy of SCO prediction and detected cancer signals across a wide diversity of cancers. The findings support the idea that the blood-based MCED test can complement existing single-cancer screening tests.
“This MCED test is meant to complement, and not replace, existing screening tests,” the research team said. “This test may also provide a complimentary screening option for individuals ineligible for or noncompliant with current screening tests, as well as for underserved communities with poor access to screening facilities.”

