Cellid released the results of a phase 2a trial of Covid-19 vaccine candidate AdCLD-CoV19.
The company disclosed cases of the adverse events partially and said the middle dose group and the high dose group did not demonstrate a statistically significant difference in neutralizing antibody titer.
The company also said it planned to conduct a separate study early next year to fight the Delta variant.
Cellid CEO Kang Chang-yul presented the results of the phase 2a study of AdCLD-CoV19 at a symposium at the Seoul National University Hospital on Tuesday.
Cellid’s investigational vaccine is a virus vector vaccine for one-shot vaccination, similar to Janssen’s Covid-19 vaccine.
Upon completing the phase 2a trial, Cellid said in June that it would newly develop AdCLD-CoV19-1, a revised version of the virus vector vaccine, for mass production. In addition, the company obtained the regulatory nod for a phase 1 study of AdCLD-CoV19-1.
Phase 2a trial fails to prove neutralizing antibody titer difference between middle, high doses
In the phase 1 study, Cellid set three groups of 10 participants for different doses – low, middle, and high. After confirming the candidate vaccine’s safety, the company set two doses – middle and high -- for a phase 2a study.
According to Kang, the company collected safety data for seven days after vaccination, and a data safety monitoring board (DSMB) decided that all doses were usable for a phase 2a study.
Using the safety and immunogenicity data during 28 days after vaccination, the DSMB held another meeting to set the middle dose and the high dose for the phase 2a trial, he said.
Cellid enrolled 120 participants for the phase 2a study and divided them into 60 for the middle dose and 60 for the high dose. Then, the company collected safety and immunogenicity data up to eight weeks after vaccination.
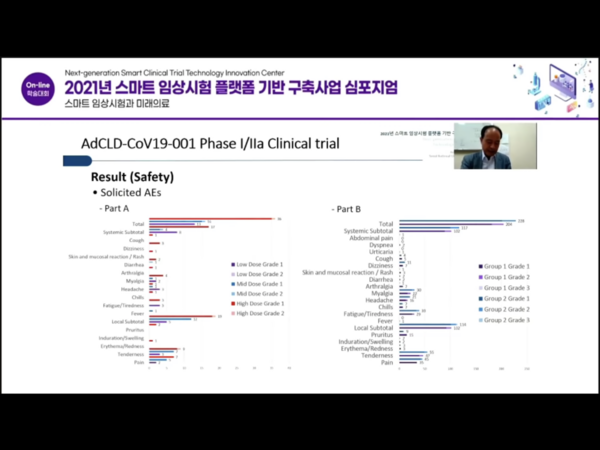
Releasing the phase 2 trial results, Cellid disclosed only solicited adverse events and not unsolicited adverse events.
According to Kang, all solicited adverse events for seven days after vaccination were grade 2 or lower. The frequency of adverse events increased along with the dose increase, and many of the side effects were local instead of systemic, he said.
In the phase 2a study with a larger number of participants, a grade 3 adverse event occurred in the high dose group, but the incidence of overall adverse events was not much different, he added.
Kang went on to say that neutralizing antibodies were produced in all of the trial participants in the phase 1 study, and the neutralizing antibody was twice as high in the middle and high dose groups compared to the low dose group.
“In the phase 2a trial, we observed that the high dose group had 1.4 times higher neutralizing antibody titer than that of the middle dose group. But the difference was not statistically significant,” Kang said.
Cellid to apply for vaccine trial against Delta variant early next year
In a Q&A session, he was asked whether Cellid’s Covid-19 vaccine development could hit a snag due to trials of a revised vaccine candidate.
“The phase 1 study will start soon, and we can finish it quickly. Then, without much impact on overall trials, we will proceed with our original schedule,” Kang said. “We’re not only pushing for local phase 2b and phase 3 studies but preparing a protocol to conduct a trial in Vietnam and get the WHO’s approval.”
He also received a question as to whether the company could skip a phase 2a trial if the results of the phase 1 trial of the new vaccine candidate produce the same or better outcomes than those of the existing candidate and proceed to a phase 2b or phase 3 study.
Kang said the company had requested the Ministry of Food and Drug Safety’s wavering of a phase 1 trial of the new candidate for a long time, but the MFDS refused to do so.
The MFDS said if the company conducts a phase 1 study on 40 participants, the regulator could review entering phase 2b and phase 3 trials, he added.
Asked how much the vaccine candidate was effective against virus variants, Kang said the vaccine showed “good effect” against most variants, including Delta.
However, the efficacy might go down over time, he said.
The company has been conducting various tests on the vaccine against many variants since early this year.
“We have completed animal tests of the vaccine against the Delta variant, and they produced good results. So we’re preparing to enter a human trial early next year,” Kang said.

