EOFlow, a company specializing in wearable drug infusion devices, continues to sophisticate its existing line of insulin pumps and expand to other areas.
The company decided to invest 18 billion won ($15.3 million) to upgrade plants manufacturing EOPump, a key material for making its wearable insulin pump EOPatch.
EOFlow explained that it received funding from the Korea Development Bank to remodel new plants for EOPump and establish production automation.
EOPump is a core driving unit applied to the company's current flagship product EOPatch and other future growth engines, including non-insulin wearable drug delivery platforms and wearable artificial kidneys.
The recent investment was a preemptive move to respond to the expected surge in demand for the product.
"New investment in EOPump is intended to internalize the production infrastructure for key driving parts to meet the future demands in countries, including Europe, China, and Americas, and we aim to manufacture more than one million a month,” CEO Kim Jae jin said.
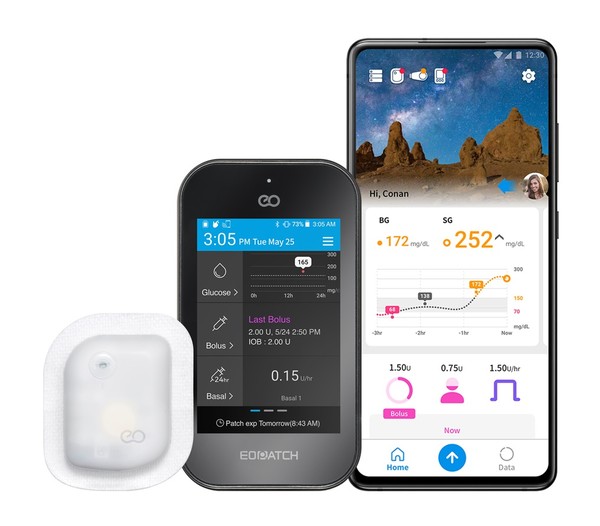
EOPatch is the world's second wearable insulin pump that continuously delivers insulin to patients. Users can link it to Narsha, a mobile application pairing with EOPatch, allowing patients to control the amount of insulin infusion and monitor blood glucose levels in real-time.
The company developed its wearable insulin pump and linked up with the application to improve the quality of diabetes management by helping patients receive and manage insulin treatment anytime and anywhere through their smartphones.
In addition to the insulin pump, EOFlow also made a wearable artificial pancreas and aimed to receive approval from the Ministry of Food and Drug Safety this year. The company's next-generation product automatically injects insulin according to the blood glucose level in connection with the continuous glucose monitoring system (CGMS).
EOPatch was authorized in Europe in May 2019. The company signed an agreement with Italian pharmaceutical company Menarini to exclusively supply it in Europe. The 150 billion-won contract will be effective for five years.
The company explained that it plans to launch EOPatch in Europe before the year ends to enter the global market successfully. It is integrating much of its capabilities for the smooth production and supply of its products.
Insulin pumps are not the only products that EOFlow is showing interest in. It established a subsidiary company Pharmeo to develop and discover various non-insulin drugs to be applied to the innovative drug delivery platforms and secure new growth momentum.
With Pharmeo, EOFlow plans to develop various other medical products, such as non-insulin medicines for subcutaneous injection and wearable artificial kidneys.
"We aim to speed up developing and marketing our wearable artificial pancreas as it could be a global product in the market as such system has not been commercialized in Korea and elsewhere," an EOFlow official said. "We think the commercialization period is getting closer as our products were receiving support from regulators when we needed governmental help."

