The Ministry of Food and Drug Safety said Wednesday it has approved 70 cases of bioequivalence testing for generic drug development in the first half, a 49 percent increase from the same period last year.
Most of the approved trials were for the drug development for chronic illnesses such diabetes and cardiovascular diseases.
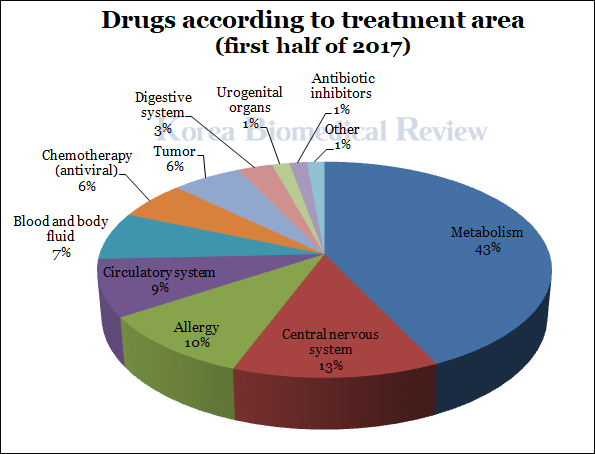
The ministry’s data also showed that 30 approved cases (42.9 percent) were for diabetes and other metabolic treatments, followed by central nervous system drugs such as antidepressants (9 cases, 12.9 percent). These two categories made up for more than half of the total with 39 cases (55.8 percent.)
Allergy medications came in third with 7 cases, and circulatory system drug came in last with 6 cases (8.6 percent).
The ministry also found that out of the 70 approved cases, 40 (57.1 percent) involved developing a generic for an original that was facing patent expiration or finishing a “re-examination” process. The re-examination process entails follow-up data tracking and research for a drug after it hits the market for four to six years to check its safety and efficacy.
The ministry said companies were continually developing generics for diabetes combination therapies such as linagliptin/metformin currently finishing the re-examination process or generics of rhinitis treatment, bepotastine besilate, which is going off patent this December.
When classified by ingredient, the ministry said 13 generics centered on R-Thioctic acid tromethamine (a compound used to mitigate diabetic neuropathy) while four comprised of apixaban.
“We expect the development of generic medications for chronic illness to grow in the future and will expend efforts to increase quality and foster development of superior generics through international regulatory harmonization of generic drugs,” the ministry said in a statement.

