Daewoong Bio said Thursday it will immediately appeal to the Supreme Court regarding the latest decision by the Patent Court that its trademark of Gliatamin, a generic pill of Gliatilin developed by Italfarmaco, was similar to that of the original drug, a cognitive improvement pill.
The statement came after the pharmaceutical company lost a lawsuit filed by Italfarmaco, which claimed that Daewoong Bio’s 대웅바이오 trademark should be nullified at the Patent Court.
Contrary to the Intellectual Property Trial and Appeal Board’s earlier decision that and Gliatamin have no brand similarity, the Patent Court ruled in favor of Italfarmaco and accepted the trademark invalidation claim.
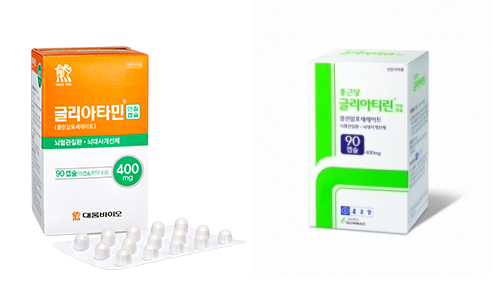
Italfarmaco claims that Daewoong Bio's Gliatamin packaging and class of goods were similar to Gliatilin and that Daewoong Pharmaceutical, an affiliate firm of Daewoong Bio, released a similar trademark even though they were aware of the contractual relationship as the company had been previously selling Italfarmaco’s Gliatilin.
Daewoong Pharmaceutical 대웅제약 had been exclusively selling Gliatilin until 2015.
However, Gliatilin's rights were transferred to Chong Kun Dang 종근당 in 2016, prompting Daewoong Pharmaceutical to sell the generic Gliatamin through Daewoong Bio.
Chong Kun Dang is currently selling the drug under the name “Chong Kun Dang Gliatilin.”
“Gliatamin and Gliatilin have no similarities in appearance, title, or concept,” a Daewoong Bio official said.
“’Glia’ in Gliatamin and Gliatilin is a medical term that refers to nerve cells and are not subject to discrimination,” a company official said. “Therefore the only difference in the trademark lawsuit involves the parts of ‘tamin’ and ‘tilin,’ which is easily distinguishable.”
Daewoong Bio claims doctors and pharmacists, who are the ones that come across both medicines frequently, can easily distinguish the difference.
“The court’s decision has an error in extending the scope of interpretation of the trademark to the general public,” the official said. “This will likely cause great confusion in naming pharmaceuticals for the industry in the future.”
The official also added that the Korean market does not sell Italfarmaco’s original Gliatilin and that it was unrealistic that Gliatamin would be confused with Gliatilin.
Meanwhile, Daewoong Bio saw outpatient prescriptions of Gliatamin reaching 29.4 billion won ($26 million) in the first half of 2017, according to a report released by Ubist, a medicine market research firm.
It surpassed the sales of the original as Chong Kun Dang Gliatilin’s outpatient prescriptions marked 22.1 billion won.

