A Korean pediatrician disclosed the first data of local patients with spinal muscular atrophy (SMA) treated with Novartis’ gene therapy Zolgensma (onasemnogene abeparvovec-xioi).
The data shows the effect of switching to Zolgensma in patients who have been treated with Spinraza (nusinersen). The switching means replacing Spinraza maintenance therapy with a single administration of Zolgensma, which could not only improve treatment convenience but save the government’s health insurance costs.
Professor Lee Seung-bok at the Pediatrics Department of the Seoul National University Hospital presented short-term data from a local trial of Zolgensma at a virtual conference hosted by the Korean Pediatric Society on Oct. 21-22.
The data include the short-term safety and efficacy of Zolgensma in six Korean patients who received Zolgensma through Novartis’ patient support program, as well as their baseline characteristics.
Five of them had type-1 SMA and the other, type-2. Their ages varied from seven months to 24.5 months when they received Zolgensma. Most notably, all six patients had already been treated with Spinraza four or five times before Zolgensma administration. Thus, the data is the first in Korea to demonstrate Switching from Spinraza to Zolgensma.
To evaluate the safety of the switching, the research team conducted complete blood counts, liver function tests, and cardiac enzymes tests after administering Zolgensma.
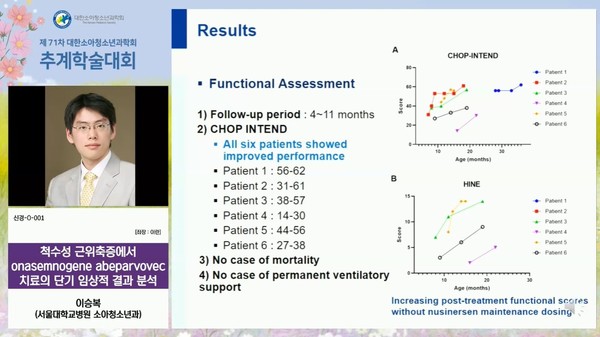
To assess motor function, the team also performed the Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND) and Hammersmith Infant Neuromuscular Test (HINE).
In Korea, Zolgensma is indicated for SMA patients with bi-allelic mutations in the survival motor neuron 1 (SMN1) gene or three or fewer copies of the SMN2 gene.
In the study, five patients had type-1 SMA with two copies of the SMN2 gene, and they were in serious conditions. The other patient, who had type-2 SMA, had three copies of the SMN2 gene. The type-2 SMA patient was aged 24.5 months at the time of Zolgensma administration, and the patient had the longest delay of treatment.
Blood tests showed that white cell and neutrophil counts, always mentioned in Zolgensma’s safety profile, tended to decrease up to two weeks after Zolgensma administration.
At week 1, monocyte increase and platelet decrease hit the highest and lowest level, respectively, but they gradually improved over time.
Levels of liver enzymes AST, ALT, and GGT also rose for a certain time after Zolgensma use, but most patients recovered at around day 50. Among the six patients, the type-2 SMA patient who was the oldest showed the steepest increase in liver enzymes.
During the follow-up of four to 11 months, all six patients’ motor functions improved. In addition, there was no death during the follow-up, and no patient required permanent mechanical ventilation.
This means that SMA patients who switched to Zolgensma had a continuous improvement in motor function without the maintenance therapy of Spinraza. However, the motor function improvement was the slowest in the type-2 SMA patient, signaling that the earlier treatment starts, the greater treatment effect.
“The short-term clinical results of AVXS-101 (Zolgensma) showed that adverse reactions in blood tests and liver function tests were normalized within two to three months after the drug administration, and the severity of adverse reactions varied depending on the patient,” Lee said. “There was no meaningful side effect or complication in Zolgensma-treated patients.”
Novartis anticipates that the latest study could positively affect the regulator’s review of granting health insurance benefits for Zolgensma.
The company had sought marketing approval and reimbursement simultaneously.
However, the Health Insurance Review and Assessment Service (HIRA) has not even started discussing Zplgensma reimbursement, although it received Novaris’ application more than six months ago.
Industry watchers also pay attention to how broad the reimbursement, if any, will be. Spinraza is covered by health insurance if the patient’s symptoms appear before three (36 months). The reimbursement is based on age, not the severity of the disease. Thus, the scope of Zolgensma reimbursement could be limited according to age.
Also, whether the HIRA will allow reimbursement for patients who switched to Zolgensma draws attention. Many real-world studies have presented data to show the effect of switching from Spinraza to Zolgensma.
Some argue that switching to a single gene therapy Zolgensma from Spinraza will be beneficial to save health insurance costs because Spinraza requires maintenance therapy costing millions of won a year.
However, it is uncertain whether the local regulatory agency, which prefers randomized controlled trial results the most, will recognize real-world evidence (RWE).
As the HIRA put off a review of Zolgensma reimbursement, families of SMA patients started to demand the reimbursement by petitioning on the Cheong Wa Dae website.
In October, lawmakers also mentioned this issue at the National Assembly’s audit of the Ministry of Health and Welfare.
At the audit, Health and Welfare Minister Kwon Deok-cheol said the government would actively consult with Novartis to set the reasonable price of Zolgensma through the risk-sharing agreement (RSA) and help patients get the drug’s benefit.
Whether his comment will speed up the review of Zolgensma reimbursement draws attention.

