Korean manufacturers of Covid-19 testing devices are actively exporting their products to the U.S.
Industry officials said the U.S. demands for Korean test kits would increase for a while due to the continued spread of the Omicron variant.
Humasis said on Monday in a regulatory filing that it signed a deal with Celltrion to supply $43.5 million worth of antigen self-test kits to the U.S. The size of the deal is 112 percent greater than Humasis’ annual revenue of 45.7 billion won ($38.5 million) last year.
The supply contract for the U.S. market will be valid until Feb. 28, 2022. Humasis, which co-developed Covid-19 antigen diagnostic kits with Celltrion, manufactures Celltrion’s Covid-19 testing kits.
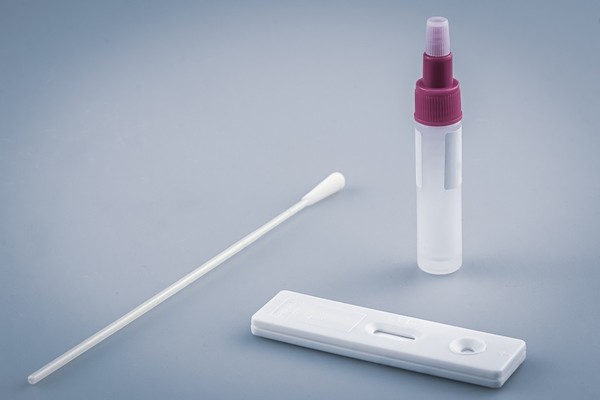
The large-scale supply deal came after two companies’ DiaTrust COVID-19 Ag Home Test received FDA approval for emergency use in October.
Celltrion, which distributes Covid-19 diagnostic devices in the U.S. through Celltrion USA, said it planned to supply DiaTrust COVID-19 Ag Home Test through online and offline distributors.
On Monday, SD Biosensor also said its Covid-19 self-test kit COVID-19 At-Home Test obtained emergency use authorization from the FDA. In addition, the company formed a strategic partnership with Roche to enter the U.S. market in earnest.
SD Biosensor supplies COVID-19 At-Home Test exclusively for Roche as SD Biosensor is the original design manufacturer (ODM). According to SD Biosensor, the home test kit offers 95.3 percent sensitivity and 100 percent specificity.
SD Biosensor CEO Heo Tae-young said, “The U.S. demand for self-test kits is spiking as Covid-19 cases are rising due to the spread of the Omicron variant.”
Another diagnostic kit maker Access Bio, which won emergency use approval for its self-test kit in the U.S. earlier, also predicted that test kit sales in the U.S. would grow rapidly. The U.S. government is distributing Covid-19 home test kits for free because home tests should be done extensively, the company said.
Access Bio emphasized expanding the sales network across the U.S. through major drugstores and online sites, including Rite Aid, Walgreens, Walmart, Target, Wegmans, and Amazon.
“After we won FDA nod for emergency use in August, we started selling the kit in October. In just a month in November, sales of home test kits surpassed those of professional diagnostic kits,” an official at Access Bio said. “The sales of home test devices will accelerate next year.”
The official went on to say that the global supply of Covid-19 diagnostic kits was in shortage because the world needs massive screenings rather than customized tests.
“This is why the U.S. government is supporting home tests through various policy measures,” he added.

