Ono Pharma Korea and Bristol-Myers Squibb said Wednesday that their blockbuster immunotherapy gained five new indications for Opdivo, soothing outcries from cancer patients protesting the government’s ban of off-label prescriptions.
■ Related : Cancer patients protest policy limiting off-label use
“We got regulatory okays for five more indications last month in Korea, and will work to become the hope for Korean cancer patients,” said Ono Pharma Korea CEO Kunihiko Ito.
Opdivo (ingredient: nivolumab) has won approvals to treat renal cell carcinoma, bladder cancer, head and neck cancer, and Hodgkin lymphoma as a monotherapy, as well as treat melanoma in combination with Yervoy, BMS said.
■ Related : Opdivo proves competence among local immunotherapies
The professors who spoke at the press conference mentioned several clinical trials – CheckMate-067, CheckMate-003, CheckMate-141, CheckMate-025, CheckMate-275, CheckMate-039 – during their joint news conference in downtown Seoul Wednesday to exemplify how the drug had proven effective in extending patients’ OS in multiple cancers.
Statistics show about 24,000 patients were diagnosed with non-small cell lung cancer in Korea in 2014 alone, far outnumbering other cancers in yearly diagnoses, according to Professor Kang Jin-hyoung of St. Mary’s Hospital
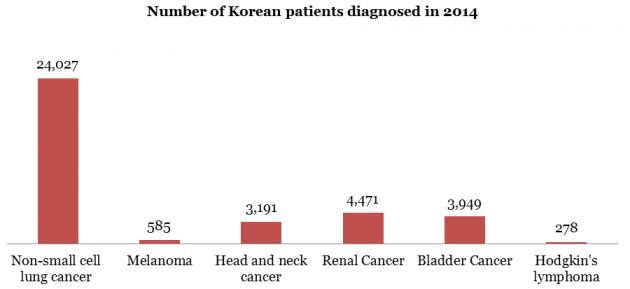
The five-year survival rate for most of these patients is about 5 to 10 percent, he added, emphasizing that PD-1 immunotherapies such as Opdivo, are opening a new era in cancer treatment.
Cancer patients are heralding the expanded indications as good news for these cancer patients who can now be able to obtain their drug – albeit at their own medical expense – without having to wait for a three-month Health Insurance Review and Assessment Service review.

Despite the progress, however, there is still a long way to go, Professor Kang stressed, due to the lack of compiled data on side effects that arise from the drug.
“The government needs to create a systematic solution to keep tabs on the side effects of these drugs,” Kang said. “We need to gather real-life clinical data, and that needs to happen through an institution that lets us comprehensively manage data from treating these diseases. Through education, we can create a treatment guideline [that will better benefit patients].”
Opdivo gained insurance coverage on Aug. 21 along with Merck & Co’s PD-L1 inhibitor Keytruda. The two have been engaged in a fierce competition to expand domestic market share.
The drug carries a price tag of 1.3 million won ($1,150) per 100mg, or 331,700 won per 20mg while its competing drug Keytruda costs 2.8 million won ($2,461) per 100mg, according to the Ministry of Food and Drug Safety.
■ Related : 2 immuno-oncology therapies receive insurance coverage
Opdivo has also been designated to treat multiple cancers by the National Comprehensive Cancer Network (NCCN) guideline, which had remained unchanged for over 20 years until the advent of PD-L1/PD-L inhibitors, the professor added.

