The biosimilar market is drawing much attention, especially after Celltrion and Samsung Bioepis became one of the leaders in the global biosimilar market.
Other Korean pharmaceutical and biotech companies – such as Chong Kun Dang, Dong-A ST, LG Chem, Alteogen, and Prestige Biopharma – are pushing to commercialize biosimilars, too.
Despite the active development of biosimilars in Korea, doctors here are still at a toddler’s stage in prescribing biosimilar drugs.

Korean clinicians still question why they should prescribe biosimilars to patients.
It is because reimbursed original drugs and biosimilars do not have a large difference in prices.
The situation in Korea is quite the opposite of the EU, where biosimilar prescription is encouraged with incentives.
In February, Seminars in Arthritis and Rheumatism published a study titled, “The cost savings of biosimilars can help increase patient access and lift the financial burden of health care systems.”
According to the study, authored by Tore Kvien, Professor Emeritus of Rheumatology at the University of Oslo and others, the U.K.’s National Institute for Health and Care Excellence (NICE) instructs British rheumatologists to begin treatment using the least expensive option.
Belgium and Germany use quota systems that drive doctors to prescribe biosimilars to up to 40 percent of their patients. Norway offered financial incentives to health systems to switch to biosimilars, raising the market shares of epoetin and filgrastim biosimilars to 80 percent.
However, incentives alone cannot induce the prescription of biosimilars because physicians and patients still worry about the biosimilar prescription.
In health conferences in Korea, doctors often say that using a biosimilar feels like “prescribing a fake medicine.”
Nocebo effect is another hurdle that biosimilars have to overcome. Nocebo effect refers to a drug failing to work because of a patient’s distrust or worries about side effects. This can happen if biosimilar manufacturers fail to improve patients’ trust in biosimilars.
To quell such worries, Celltrion and Samsung Bioepis have steadily emphasized that switching from original drugs to biosimilars is safe in the long term.
Then, what kind of cautions do European physicians take when prescribing biosimilars?
Korea Biomedical Review met with Paul Emery, a professor of rheumatology at the University of Leeds, during the 2022 European Alliance of Associations for Rheumatology (EULAR) Congress, held from June 1-4 in Copenhagen, Denmark.
Emery asked how European doctors prevent the nocebo effect in biosimilars, they “ensure the patient’s autonomy.”
“It is important to give patients the right to choose, with sufficient explanations. We also inform them that they can change to the original drug, after switching to a biosimilar, at any time,” Emery said. “Some patients returned to the original drug, but most patients maintained biosimilar prescriptions.”
In Korea, rheumatologists started to discuss biosimilar prescriptions actively.
Doctors shared the latest information on biosimilars at the 42nd annual meeting of the Korean College of Rheumatology (KCR 2022) held from May 19-21.
Professor Kim Jin-seok of rheumatology at Jeju National University Hospital said there was no significant difference in prices of the original drug and a biosimilar from the patient’s point of view.
“Biosimilars need to have more convenient features such as less painful injections, less dose, or less drug leaking out of blood vessels,” Kim said.
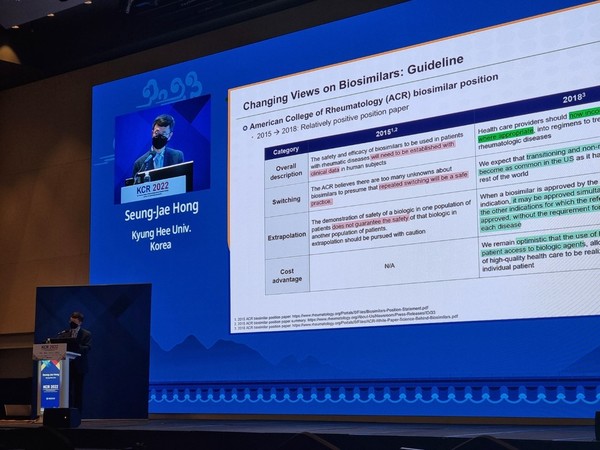
Professor Hong Seung-jae of rheumatology at Kyung Hee University said that physicians should provide more information for patients to reduce the nocebo effect when prescribing a biosimilar.
“Using the real-world data, doctors need to be more confident about biosimilar prescription,” he said.
He also urged the government to increase state support for biosimilar prescriptions.

