PARIS – A Korean expert has given a positive evaluation to the phase 3 clinical trial results of VEGFR2 Tigit Tyrosine Kinase Inhibitor (TKI), “rivoceranib,” which proved effective in the first-line treatment of liver cancer combined with the anti-PD-1 immuno-cancer drug, "camrelizumab."
Rivoceranib and camrelizumab are new drug candidates, each developed by HLB of Korea and Jiangsu Hengrui Medicine of China.
Professor Lim Ho-young of the Department of Hemato-oncology at Samsung Medical Center, noting that rivoceranib is the first TKI that successfully treated liver cancer combined with an immune-cancer drug, would emerge as be a useful alternative therapy for patients who cannot the current first-line treatment, bevacizumab, due to high risk of bleeding.
At the European Society for Medical Oncology (ESMO) 2022 held in Paris on Saturday, the open-label phase 3 clinical trial results of the combined use of camrelizumab and rivoceranib in the primary treatment of liver cell cancer, compared to the “sorafenib” monotherapy.
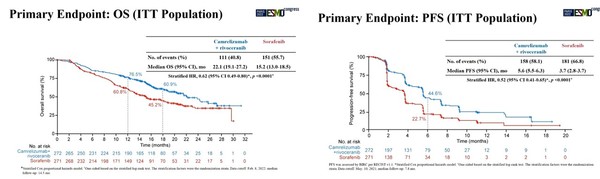
In the study, the camrelizumab+rivoceranib group’s median overall survival (mOS) was 22.1 months compared to the control group’s 15.2 months, lowering the death risk by 38 percent. The median progress-free survival (mPFS) also stood at 5.6 months vs. 3.7 months, reducing disease progress and death risk by 48 percent.
Besides, the overall response rate (ORR) of the camrelizumab+rivoceranib group was 25.4 percent, improving from the control group’s 5.4 percent. The incidence of third- and fourth-grade treatment-related adverse reactions reported in the camrelizumab+rivoceranib group was 80.5 percent, and the ratio of steroid use was 16.2 percent.
Professor Lim, after listening to the presentation at the ESMO 2022 conference, said, “The study is meaningful because another combination treatment has attained success in the primary treatment of hepatocellular carcinoma. Moreover, it was the first study that succeeded by combining immune-oncology (IO) and TKI.”

Noting that the overall survival result of the camrelizumab and rivoceranib combination was “very positive,” Lim said it would provide an alternative therapy for patients who cannot use bevacizumab due to high risk of bleeding.
Currently, the combination of tecentriq (ingredient” atezolizumab) and bevacizumab has established itself as the standard treatment for the primary cure of hepatocellular carcinoma. However, due to bevacizumab’s adverse effect on bleeding, patients with a high risk of bleeding can only use TKI monotherapy, including Lenvima (ingredient: lenvatinib).
If the U.S. Food and Drug Administration approves the camrelizumab and rivoceranib combination based on such usefulness, the combination therapy also will likely win approval without much difficulty at home, according to Professor Lim.
“However, it is not easy to regard the phase 3 clinical trial as a global study,” he said. “Most subjects were Asians -- ethnic Chinese from China, Taiwan, and Hong Kong.”
As a matter of fact, 83 percent of the subjects in the camrelizumab and rivoceranib study were Asians, and only 17 percent were non-Asians.
Lim added that the pathogenetic characteristics of the patients in the study might have affected maximizing the camrelizumab+rivoceranib combo therapy’s effectiveness.
“About 75 percent of patients in the study had liver cancer caused by hepatitis B virus,” the SMC professor pointed out. “Considering the widespread theory that the control drug sorafenib is more effective in hepatitis C virus patients than in hepatitis B virus patients, the combination of camrelizumab and rivoceranib might have maximized the effectiveness of the study participants.
Lim also noted that the ratios of third- or fourth-grade adverse effects reported in the camrelizemab and rivoceranib combination appeared a little too much compared to the control group, adding that it would be necessary to watch other study results concerning high blood pressure and lowered liver function related to rivoceranib.
Lim also threw a question mark regarding the domestic approval of the camrelizumab+rivoceranib combination and its possibility of getting insurance benefits.
“Currently, the combination of atezolizumab and bevacizumab is receiving reimbursement. Therefore, for the new combination therapy to receive reimbursement, it must show better results in terms of price or adverse effects,” he noted. “I am not very certain whether the camrelizumab and rivoceranib combo therapy will persuade the authorities regarding these points.”
Related articles
- HLB confirms rivoceranib’s safety in phase 1 gastric cancer trial
- HLB to release phase 3 results of cancer drug candidate at ESMO 2022
- HLB’s rivoceranib also shows efficacy in esophageal cancer
- HLB to apply for FDA nod for rivoceranib early next year
- [ESMO 2022] Lung cancer treatment develops from strength to strength – except for Korea?
- HLB set to apply for conditional domestic approval of rivoceranib

