The U.S. Food and Drug Administration has approved “FreeStyle Libre Flash Glucose Monitoring System,” which can be used by adult patients to make diabetes treatment decisions without calibration using a blood sample from the fingertip.
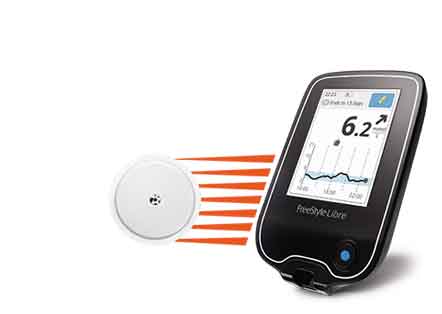
Developed by Abbott, the system reduces the number of blood samples collected from fingertips by inserting a small sensor wire below the skin. The sensor continuously measures and monitors the glucose levels of the user. The system is intended for the use of people over 18 years with diabetes. After a 12-hour startup period, it can be worn for up to 10 days.
“The FDA is always interested in new technologies that can help make the care of people living with chronic conditions, such as diabetes, easier and more manageable,” said Donald St. Pierre, acting director of the Office of In Vitro Diagnostics and Radiological Health. “This system allows people with diabetes to avoid the additional step of fingertip calibration, which can sometimes be painful, but still provides necessary information for treating their diabetes—with a wave of the mobile reader.”
According to the Centers for Disease Control and Prevention, more than 29 million people in the U.S. have diabetes. People with diabetes either do not make enough insulin (type 1 diabetes) or cannot use insulin properly (type 2 diabetes). When the body doesn’t have enough insulin or cannot use it effectively, sugar builds up in the blood, which can lead to heart disease, stroke, blindness, kidney failure, or the amputation of toes, feet or legs.

