Genome & Company said on Tuesday that it will drop the phase-1b clinical trials of GEN-001 in solid cancer in the U.S. and Korea, citing too much competition in new drug development for solid cancer.
Instead, the company will focus on its gastric and biliary tract indications in phase-2 combination trials, it said.
GEN-001 is the first immuno-cancer microbiome candidate drug in Korea, mainly composed of Lactococcus lactis which proved effective as a monotherapy but also in combination with immune checkpoint inhibitors.
Currently, this drug is linked with Pfizer’s Bavencio (avenolumab) in phase 1/1b clinical trials in solid tumors (non-small cell lung cancer, head, and neck cancer, urothelial cancer) in the U.S. and South Korea. GEN-001 is also in phase 2 trials in combination with Bavencio for gastric cancer in Korea. The company plans to start a phase 2 clinical trial with MSD’s Keytruda (pembrolizumab) for biliary tract cancer.
However, Genome & Company CEO Seo Young-jin announced a few changes.
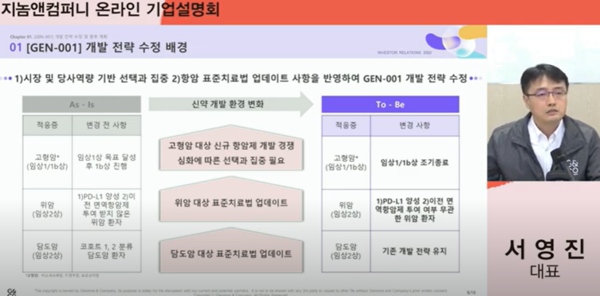
Citing fierce competition in the development of new anticancer drugs for solid cancer patients, Seo said, “We have decided to terminate the phase 1/1b clinical trial for solid cancer early to focus more on the gastric and biliary cancer trials.”
Clinical trials for gastric cancer are actively being conducted in gastric cancer patients who are PD-L1 positive and have not received previous immuno-oncology drugs. However, he explained, “Due to the update of standard treatment for gastric cancer, we have now decided to conduct clinical trials in all gastric cancer patients, regardless of whether they are PDL1-positive and have previously received immuno-oncology drugs.”
He said the company was conducting clinical trials at leading research hospitals in Korea, including Samsung Medical Center, and the studies achieved the objective response rate (ORR) primary endpoint.
Interim analysis and results will be announced early next year, he said.
He went on to say, “The standard treatment for biliary cancer has also been revised and we have already devised a clinical plan for patients who have received immuno-oncology drugs in clinical trials for biliary tract cancer to classify them into cohorts 1 and 2, so the progress of clinical trials will not be affected.”
In this regard, the company has completed the phase 1 clinical trial and confirmed its safety, and recommended the phase 2 dose (RP2D) trial.
“Based on these clinical trials, we believe we will be able to confirm the efficacy of GEN-001 as a new third-line treatment option for patients with PDL1-positive gastric cancer and as new second- or third-line therapy for biliary tract cancer,” said CEO Seo. “The current market conditions are very challenging and thus, we have announced several changes to GEN-001 and hope our investors will take note and continue supporting.”
Related articles
- Genome & Company identifies mechanism of anti-obesity microbiome action
- Genome & Company to build microbiome manufacturing plant in US
- Liveome wins patent for microbiome therapy development platform in Japan
- Macrogen wins patent for microbiome health info analysis device
- Experts to discuss microbiome therapeutics in Seoul at Microbiome Connect Asia
- Microbiome Asia to showcase Korea's rapidly growing microbiome industry
- Why Genome & Co. changes p2 trial IND for bile duct cancer drug?
- Genome&Company finds new target to improve cancer immunotherapy
- Genome & Company targets 3rd-line stomach cancer treatment with microbiom-based immunotherapy

