Bristol-Myers Squibb and Merck & Co are conducting several clinical trials in Korea for Opdivo and Keytruda, respectively, engaging in a fierce competition to expand their respective drug’s indications.
This newspaper analyzed the clinical trials taking place in Korea as of Sept. 29 for the two immunotherapies to find six clinical trials in progress for Keytruda, (ingredient: pembrolizumab) and two for Opdivo (ingredient: nivolumab).
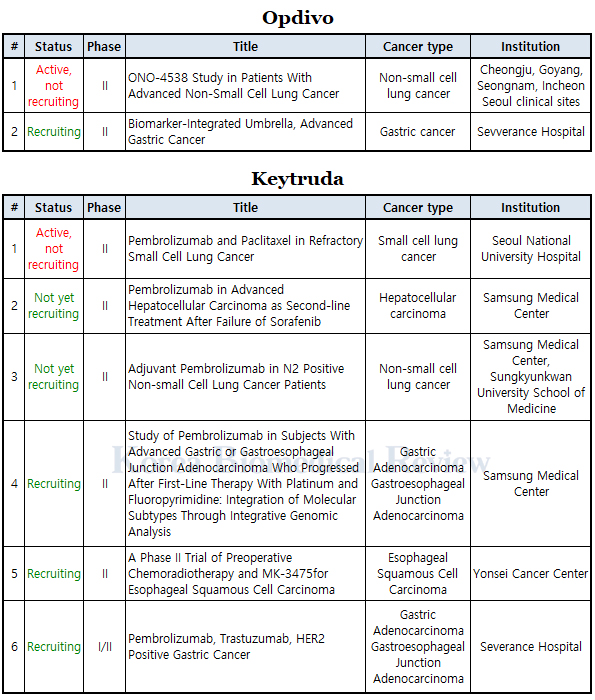
By cancer type, the number of Keytruda trials in Korea were two for gastric cancer, two for lung cancer (small cell and non-small cell), one for liver cancer (hepatocellular carcinoma), and one for esophageal cancer. Meanwhile, Opdivo had two phase 2 trials conducted in Korea for advanced non-small cell lung cancer and advanced gastric cancer.
When expanded to include the number of clinical trials taking place globally, including Korea, the number of Keytruda trials increased to a total of 32, and that of Opdivo, to 28.
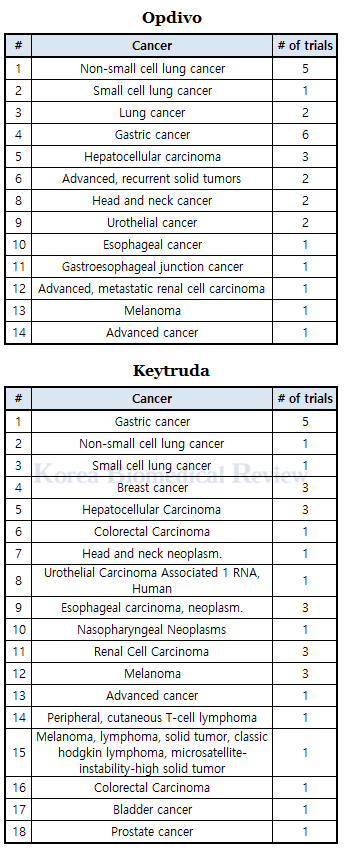
Compiled data, including trials conducted only in Korea, showed Keytruda focused on cancer in stomach (five), liver (three), breast (three), esophageal (three), melanoma (three), and kidney (three).
Opdivo trials indicated BMS’ concentration on lung cancer (eight), gastric cancer (six), and liver cancer (three), among others.
The two companies rapidly expanded its indications for their drugs last month. The Ministry of Health and Welfare approved Opdivo for seven indications in Korea as a second-line treatment while allowing Keytruda to treat melanoma and non-small cell lung cancer.
Globally, BMS Korea said Thursday the U.S. Food and Drug Administration expanded Opdivo’s indication to treat hepatocellular carcinoma in the U.S. while Japanese regulatory authorities approved the drug to treat gastric cancer.
Merck & Co’s Keytruda has also recently gained another indication to treat gastric cancer in the U.S. through the FDA.
Experts here are now predicting Opdivo and Keytruda will gain expanded indications for liver and stomach cancer domestically.
“The two immunotherapies will soon obtain indications for gastric and liver cancer,” said Professor Bang Yeong-joo of Seoul National University Hospital at a news conference held by the Korea Biomedicine Industry Association Monday.

