AstraZeneca Korea said Friday its ovarian cancer drug Lynparza began to receive insurance benefits early this month, becoming cheaper than U.S.’s list price of about $13,000 as a maintenance therapy.

The coverage of Astra’s PARP inhibitor comes two years after the Ministry of Food and Drug Safety’s initial approval in August 2015. The coverage is set to make the drug more affordable with each capsule priced at around 10,000 won ($8.8), according to Korea Pharmaceutical Information Center. Patients should take eight tablets twice a day.
Two clinical trials -- Study 19 and SOLO-2 -- have proved the safety and efficacy of the drug. The Korean government’s approval was based on Study 19 findings for which progression-free survival (PFS) was the primary endpoint, company officials said.
Study 19 pitted Lynparza (ingredient: olaparib) against a placebo in 265 patients to find the olaparib increased the patients’ PFS by 3.6 months (8.4 months versus 4.8 months). Data analysis showed a 6.9-month increase in the Lynparza arm among ovarian cancer patients with a BRCA mutation, indicating an 82-percent decrease in the risk of disease progression, according to the company. For women without the BRCA mutation, PFS was extended notably less at 1.9 months (7.4 months versus 5.5 months).
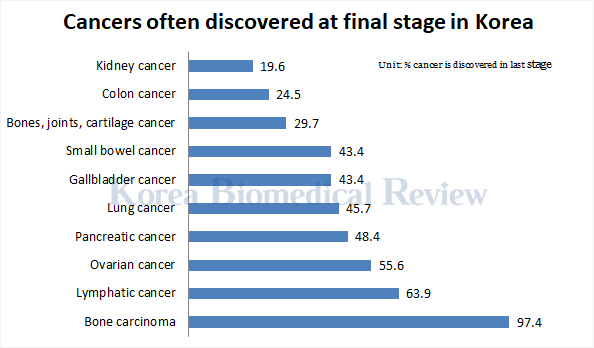
“Ovarian cancer is a silent killer often discovered at the last stage and does not respond well to treatment,” said Professor Kim Yong-man who spoke at AstraZeneca Korea’s press conference Wednesday. “It was difficult as a physician to explain to the patients and their families that the drug would cost around 3 million won a month. The insurance coverage will now ease some of the financial burdens.”
In the United States, AstraZeneca got some surprisingly good news. Two months ago, the FDA approved Lynpaza to treat women with ovarian cancer regardless of BRCA status, based on phase-3 SOLO-2 trial. Experts have noted the broad approval is indicative of FDA Commissioner Scott Gottlieb’s liberal stance toward approving drugs.
AstraZeneca Korea officials said that while they have applied for the same approval to the Korean ministry, they are unsure whether it will give the go-ahead.
The company has also applied for the same approval to European regulatory bodies, they added.

