ViroMed said it has confirmed that VM202, an investigational gene therapy being tested for diabetic neuropathy, inhibited pain-inducing factors and controlled inflammatory responses in an animal study.
The latest test, the first to reveal the mechanism of pain reduction in diabetic neuropathy, has boosted VM202’s potential to be recognized as a disease-modifying drug (DMD), the biotech firm said.
The experimental treatment showed a different mechanism for reducing pain from those of conventional pain medications prescribed to diabetic neuropathy patients, according to the company.
In the animal study, neuropathic pain-induced animals had high levels of pain-inducing factors, but the injection of VM202 suppressed the expression of the pain-inducing factors.
ViroMed researchers administered VM202 into the muscles of chronic constriction injury (CCI)–induced mice and observed the level of pain relief.
Results showed that hepatocyte growth factor (HGF) proteins, which have therapeutic effects, were expressed in muscle tissues, dorsal root ganglia, and sciatic nerve.
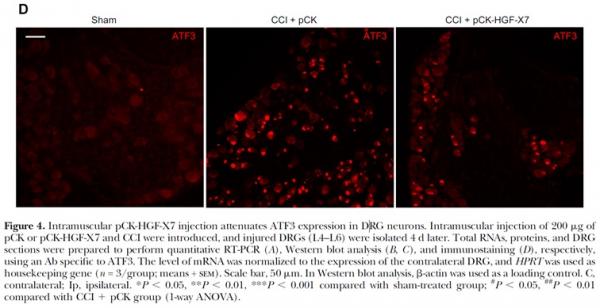
Neuropathic pain-induced animals had elevated levels of expression of factors such as CSF1 and ATF3 involved in pain induction in dorsal root ganglia, whereas such factors were suppressed in the VM202-treated group.
In the VM202-injected group, division and activation of small glial cells and astrocytes associated with neuroinflammatory responses were also inhibited.
The VM202 group was less hypersensitive than the control group, which was sensitive to physical and thermal stimuli, showing that the drug candidate can mitigate the sensitivity of the sensory nerve in neuropathic pain, the researchers said.
ViroMed said it would continue to publish study results on a correlation between VM202’s therapeutic mechanism and pain reduction.
“We could observe VM202’s excellent efficacy in long-term pain reduction in clinical trials because it had such distinctive biological actions. The results will be a scientific basis for positioning VM202 as a fundamental, effective, and safe treatment for diabetic neuropathy,” said Kim Sun-young, chief science officer of ViroMed.
The study has been published online on the Federation of American Societies for Experimental Biology (FASEB) on Tuesday.

