Roche Diagnostics Korea said Monday that it launched its high-speed slide scanner for digital pathology, called the VENTANA DP 200, in the domestic market with approval from the Ministry of Food and Drug Safety.
The device is a next-generation digital pathology scanner that connects to Roche Diagnostics’ iScan Coreo and VENTANA iScan HT.
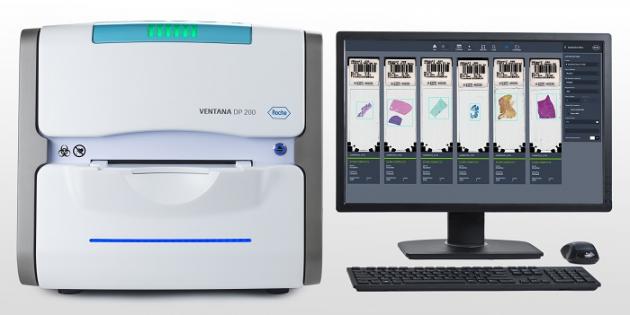
Specialist traditionally diagnosed a patient’s tissue slide by reading the microscope with the naked eye after staining during a biopsy. The advent of digital pathology scanner equipment has helped pathologists diagnose patients, anywhere, anytime, the company said.
These devices allow specialists to diagnose patients by utilizing digital images scanned from a tissue dyeing slide instead of microscopic readings.
The DP 200 is a tray-type design that does not require slides to be moved directly, reducing scan errors and adding stability to equipment operation.
The device also applies a unique International Color Consortium (ICC) color profile to each image to ensure the digital image closely matches colors observed under a microscope. It’s Area of Interest (AOI) automatic detection function produces high-quality images, according to Roche.
Roche Diagnostics has a total product portfolio for pathology diagnosis, including BenchMark Special Stains, H&E dyeing equipment HE600, and VANTAGE, a pathology management system, the company said.

