Celltrion increases supply of biosimilar product
The sales of Remsima (Infliximab), Celltrion’s biosimilar for rheumatoid arthritis treatment, are rising in the United States and Europe.
Celltrion announced Monday it signed a 56.8 billion-won ($50 million) contract with Celltrion Healthcare, a wholesaler which sells Remsima in overseas markets. The contract amount accounted for 9.41 percent of Celltrion’s recent sales.
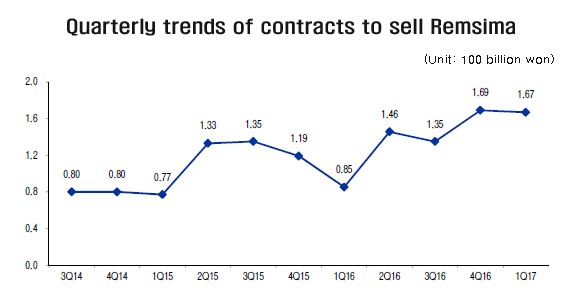
The contract amount between Celltrion and Celltrion Healthcare in the first quarter soared 95.8 percent from a year earlier, said Shin Jae-hoon, an analyst at eBest Investment and Securities, in a report released Tuesday. In the first three months of 2017, the contract to sell Remsima totaled 166.8 billion won ($1,493 million), double the comparable amount of a year ago. “It seems to reflect the rise in Remsima prescription in the United States and Europe,” Shin said.
According to the report, the supply contract with Celltrion Healthcare has been on the rise, from 80 billion won in the fourth quarter of 2014 to 119 billion won in the last quarter of 2015 and to 146 billion won in the corresponding period of 2016. The company’s turnover also increased 11 percent to 670 billion won, along with the increase in Remsima’s sale.
Its share price, however, remained at a standstill. As of Tuesday noon, Celltrion stocks were changing hands at 90,500 per share, down 1,000 won from March 28, 2016. Prices from March 20-28 also hovered around the 90,000-won range, ranging from 88,100 won to 90,500 won.

