
Bridge Biotherapeutics, also known as BridgeBio, said Thursday it presented the results of a preclinical trial on BBT-877, an investigational idiopathic pulmonary fibrosis treatment, at the IPF Summit 2018 in San Francisco, which kicked off on Monday.
Bridge Biotherapeutics is a Korean biopharmaceutical firm specializing in new drug development.
LegoChem Biosciences first discovered BBT-877 and licensed exclusive global rights to Bridge Biotherapeutics for further development. BBT-877 targets Autotaxin (ATX), which is known to play a role in various diseases, including fibrosis, autoimmune disease, and tumors, and has served as a new target for the biopharmaceutical industry and academia.
Another biopharmaceutical firm Galapagos recently won approval to go ahead with phase 3 clinical trials and bypass the phase 2b study in the U.S. for its ATX inhibitor GLPG1690, after showing promising results from a phase 2a study on 23 IPF patients.
Bridge Biotherapeutics presented the results of the BBT-877 preclinical study at the poster session of the IPF Summit 2018, demonstrating robust efficacy in the bleomycin-induced mouse model, the company said. Results showed BBT-877 effectively reduced lung fibrosis, indicated by lower Ashcroft scores and collagen deposition (staining), compared to other drugs.
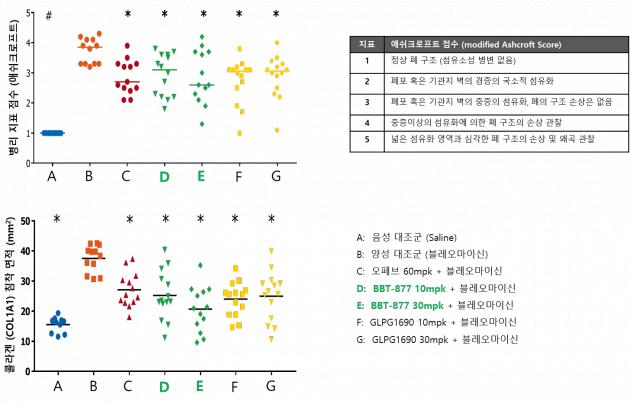
The data implies a high potential of BBT-877 to be the “best-in-class” drug for IPF treatments, the company said.
"It is a great opportunity for Bridge Biotherapeutics to present the outstanding preclinical study results on BBT-877 at the IPF Summit 2018.” Bridge Biotherapeutics CEO James Lee said. “Bridge Biotherapeutics aims to develop BBT-877 as the best-in-class drug for IPF as fast as possible to bring this investigational compound to patients as a new treatment option."
The firm is also developing the first anti-Pellino-1 compound called BBT-401 in the U.S. to treat ulcerative colitis. It aims to launch the international multicenter, first-in-patient study by the end of this year. Sungkyunkwan University and Korea Research Institute of Chemical Technology first discovered the compound and licensed exclusive global rights to Bridge Biotherapeutics in 2015.

