Number of approval for clinical trials increased 12% last year
A boom to develop biomedicines is sweeping the domestic pharmaceutical companies, government and business officials said Wednesday.
Out of the 628 approvals given to clinical trials last year, 226 cases, or 36 percent of the total, were related to biomedicines. The total number of approvals for clinical tests fell but that for biomedicine-related clinical trials rose 12 percent from 2015 to 202 cases last year.
The Ministry of Food and Drug Safety (MFDS) made these and other points Friday based on its analysis of statistics concerning the approval of clinical tests last year
The number of approvals for clinical trials totaled 387 last year, down 14 percent from 2015, but that for biomedicine-related tests rose 12 percent year-on-year to 226. The share these biomedicines, including cell therapies and biosimilars, take out of the total clinical experiments had also been on the rise, from 26 percent in 2014 to 30 percent in 2015 and to 36 percent last year, the ministry said,
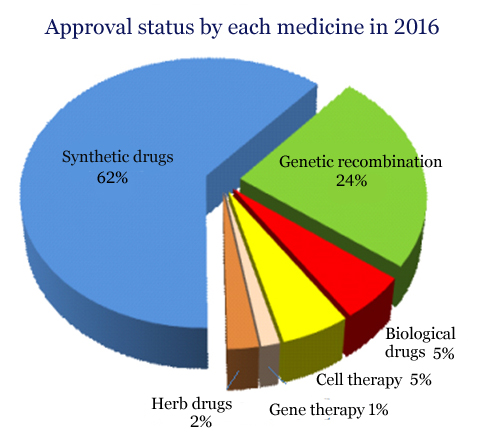
By the type of biomedicine, those related to genetic recombination topped the list of approvals, followed by biological products, including vaccines, with 33, cell therapy products, also with 33, and gene therapies, with nine.
Genetic recombination medicines dropped 4 percent from 158 in 2015, but Phase 1 clinical trial for the drugs, mostly at the early stage in development, increased 25 percent to 35, from 28 in 2015. The permission for cell therapies rose 32 percent to 33, from 25 in 2015. The ministry attributed the rise in cell therapies to heightened interests in allogeneic cell therapy that has a high potential for commercialization through mass production, compared with autologous cell therapy.
There were also changes in who conducted clinical trials, as researcher-centered clinical trials conducted for academic purpose sharply increased.
The researcher-centered clinical trials refer to those aimed at checking new indications, usage, and doses of approved medicines for academic purposes. These tests numbered only 37 in 2015 but increased 28 percent to 171 in 2016.
The increase in these trials indicates the reinvigoration of various clinical researches, including reviewing domestic medical situations, the pursuit of usage and doses for individual patients, and the research into interactions among drugs, all of which help to improve public health.
Regulators approved 457 clinical trials applied by drugmakers to develop medical products last year, down 15 percent from 2015. Domestic trials dropped 22 percent from 2015 to 190 as approvals in each step from Phase 1 to Phase 3 were down. Phase 2 clinical tests showed the steepest fall from 25 in 2016 from 42 in 2015.
Multinational clinical trials also declined. The approvals for overseas trials dropped 9 percent to 267, as both Phase 2 and Phase 3 clinical trials were down to 71 and 136, respectively. However, Phase 1 clinical tests increased 14 percent to 57, from 50 in 2015.
“Phase 1 clinical trials are the first step to apply a new drug to people and play a vital role in determining whether researchers should push ahead with its development,” a ministry official said. “The continuous increase in the number of clinical trials abroad at an early stage means global community recognizes overall conditions in Korea, such as strict regulations standard and well-organized workforce and facilities in clinical trial organizations.”
Also, the ministry has found trials concerning anticancer therapies accounted for the largest share of total tests approved. The approvals for clinical trials of anticancer therapy numbered 202, representing 76 percent of the total. Among them, 154 were related to anticancer immune therapies.
Among the domestic drugmakers, Daewoong Pharmaceutical received the largest number of approvals with 16 cases, followed by Chong Kun Dang Pharm with 14 and Dong-A ST with eight. Among multinational companies, Lilly Korea topped the list with 15, chased by Janssen Korea and MSD Korea, with 13 each.

