Korean pharmaceutical and biotechnology companies emphasized their competitiveness and unveiled latest pipelines at the 2019 J.P. Morgan Healthcare Conference, the largest healthcare industrial event in the world.
More than 485 pharmaceuticals from all over the world are participating in the annual meeting held in San Francisco from Monday to Thursday (WST). About 30 Korean drugmakers joined the event to present their latest products and business plans to investors.
Hanmi Pharmaceuticals President-CEO Kwon Se-chang, for example, presented his company’s R&D strategy and vision for this year.
Kwon stressed that the company plans to focus on the R&D for three treatments -- next-generation obesity treatment (HM15136), nonalcoholic steatohepatitis (NASH) treatment (HM15211) and acute myeloid leukemia (AML) treatment (HM43239) – in 2019. The company is also planning to make significant strides in the field of immunology in China, he said.
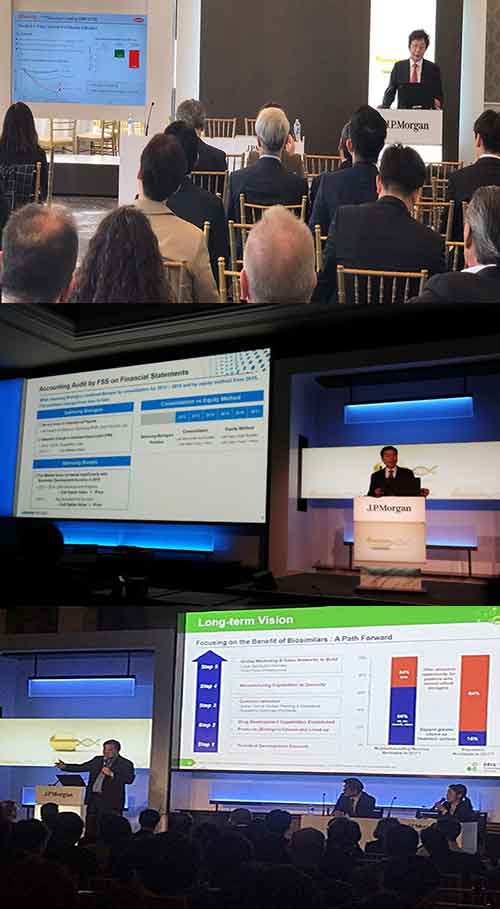
Samsung BioLogics presented its plans during the presentation entitled “Innovation and Growth of Samsung in the Biologics Industry.”
"As of January 2019, we have received a total of 41 orders, including 27 contract manufacturing organization (CMO) orders and 14 contract development organization (CDO) and contract research organization (CRO) projects,” Samsung BioLogics CEO Kim Tae-han said. “We are also in the process of negotiating orders with more than 20 companies and plan to add 10 additional CDO and CRO orders by the end of this year.”
Kim also talked about the company’s plan to raise the production capacity of its three factories to 50 percent this year. The production capacity for the three factories is currently at 25 percent.
LG Chem also introduced the current status and future strategies of its bio-business. In particular, the company announced the results of an open innovation project to expand the research and development field on metabolic diseases, anti-cancer, and immune diseases.
The company signed a strategic partnership with U.S. CUE Biopharma, U.K.’s AVACTA and Medipost to jointly develop immunotherapeutic drugs and cell therapy drugs.
Also, LG Chem has opened a global innovation center in Boston, Mass., to accelerate global clinical trials, and conduct open innovation activities for new drugs.
Celltrion, which is trying to emerge as a global biosimilar maker, was also keen on delivering its key strategies to become a company that utilizes a global direct sales system at the conference.
“Remsima SC, which applied for a European license last year, will be the main drug that helps us become a global biosimilar company,” Celltrion Chairman Seo Jung-jin said. "We plan to expand the Celltrion Group into a global biopharmaceutical company by completing a direct sales system starting with Europe after receiving the sales licensing for Remsima SC."
Seo also expressed the company’s goal to expand its business into China.
"We are actively negotiating for the establishment of a joint venture in China, and we expect to establish the venture until the end of this year,” Seo said. "We plan to make accessible biosimilars at an affordable price to Chinese patients.”
Celltrion is conducting clinical trials for Remsima after receiving approval from the China Food and Drug Administration (CFDA) in May 2017. If approved, Celltrion will become the first foreign company to have its biosimilar approved in China, Seo added.

