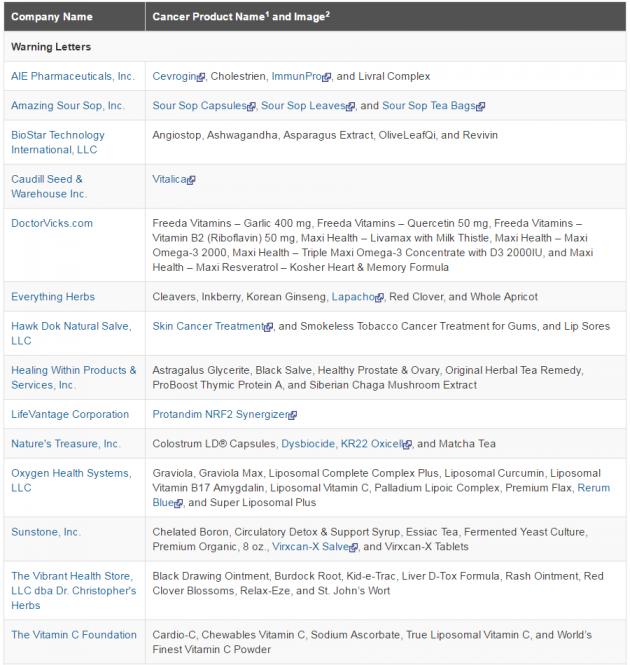
The U.S. Food and Drug Administration (FDA) posted warning letters on Tuesday to 14 U.S.-based companies, which are illegally selling more than 65 products that falsely claimed to prevent, diagnose, treat or cure cancer.
All products are marketed and sold mostly on websites and social media platforms without FDA approval.
“Consumers should not use these or similar unproven products because they may be unsafe and could prevent a person from seeking an appropriate and potentially life-saving cancer diagnosis or treatment,” said Douglas W. Stearn, an FDA director. “We encourage people to remain vigilant whether online or in a store and avoid purchasing products marketed to treat cancer without any proof they will work. Patients should consult a healthcare professional about proper prevention, diagnosis, and treatment of cancer.”
The illegally sold products addressed in the warning letters include pills, creams, ointments, oils, drops, syrups, teas and diagnostics (such as thermography devices). The products marketed for humans or pets claimed to prevent, reverse or cure cancer and killing/inhibiting cancer cells or tumors, all of which are illegal and unproven claims.
It is a violation of the “Federal Food, Drug, and Cosmetic Act” to market and sell products that claim to prevent, diagnose, treat, mitigate or cure diseases without first demonstrating to the FDA for their effectiveness and safety.
As part of its effort to protect consumers from cancer health fraud, the FDA has issued more than 90 warning letters in the past 10 years to companies marketing hundreds of fraudulent products making cancer claims on websites, social media and in stores.
The FDA has requested responses from the 14 companies stating how the violations will be corrected. Failure to correct the violations promptly may result in legal action, including product seizure, injunction, and criminal prosecution.