Medytox Korea, a subsidiary of Medytox, has licensed in and launched HNT Medical’s Carewave, Korea’s first extracorporeal shock wave equipment for erectile dysfunction approved by the Ministry of Food and Drug Safety.
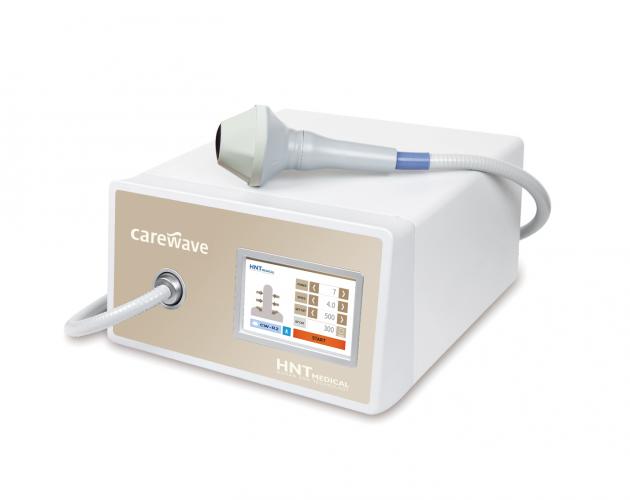
Carewave, a magnetic-type medical device, gives a weak impact on the male genital organ with low-intensity energy extracorporeal shock wave (LI-ESWT), which induces microtrauma of the cell to promote vascular endothelial growth factor expression and neovascularization and blood circulation, to help restore natural erectile function.
In particular, the patented multi-focus technology provides improved convenience for the operator, while diversifying the treatment feature.
Erectile dysfunction therapy using external shock-wave medical devices has received considerable attention overseas as an alternative treatment for improving erectile function through the creation of new blood vessels.
“CareWave, which has reasonable price competitiveness compared to the existing foreign products, is Korea’s first external shock wave medical devices to be approved for erectile dysfunction treatment,” said Oh Kyoung-seok, director of Medytox Korea’s domestic business division.
Medytox Korea will expand our market through active marketing activities as it has introduced a high-quality equipment that incorporates numerous external authentication processes and patented technology, he added.

