Ildong Pharmaceuticals is preparing to launch its chronic hepatitis B drug, Besivo (compound: besifovir dipivoxil), as the original developer’s product goes off patent in November, the company said Monday.
Chronic hepatitis B is an infection of the liver that can cause organ scarring, liver failure, and cancer. The sometimes fatal disease is prevalent in African and Asian countries; however, patients can manage the disease with proper medication.
Gilead길리어드, the current market leader in the chronic hepatitis B drug market, will lose patent rights on its blockbuster drug, “Viread,” within six months, which has spurred competing companies to release generic products in hopes of taking over the market.

Ildong일동제약 is one company that has chosen to enter the market with Besivo, an oral nucleotide drug, which has proved non-inferiority in phase 3 clinical trials compared to Gilead’s Viread Tab, company officials said at a news conference.
“Safety is our biggest advantage. Our drug has little kidney toxicity and less bone mineral density reduction, which will make Besivo the safest drug out in the market,” said Ahn Sang-hoon, a professor at Yonsei Severance Hospital, who took the lead in conducting the clinical trials. “Considering that chronic hepatitis patients must take medication for five to 10 years, it’s critical that the drug is safe to take for an extended period.”
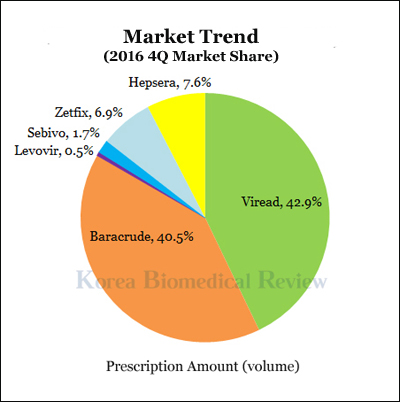
However, industry experts have noted Besivo may have difficulties finding a foothold in the competitive market, as patients must deal with two inconveniences when taking the Besivo Tab – the large size of the tablet, and the decrease of an amino acid called L-carnitine.
The Besivo Tab is 17.0-mm wide, making it almost double the size of Gilead’s Viread Tab. Company officials, who had once considered advising patients to cut the medication in half, said that “although the size is relatively bigger, we have noted it does not pose many problems in taking the pill.”
Company officials also addressed the issue of the drop in a naturally produced amino acid called L-carnitine, which requires patients to supplement Besivo with another drug produced by Ildong called “L-Carn.” Ahn noted that although patients in the clinical trial had to take two tablets of L-Carn, the third clinical trial revealed the need to take only one. “Taking one additional pill is a minor inconvenience. We believe that the advantages of having Besivo outweigh having to take one more pill,” he said.
The company will likely see an increase in L-Carn sales once Besivo goes on the market, industry experts noted. The company also has the upper hand in price negotiations at the home market as the drug is made and distributed locally, according to company officials. Ildong Pharmaceutical’s marketing official said that price negotiations are currently underway to work out whether the company will sell Besivo along with L-Carn Tab at a discounted price, and is also preparing to export the drug to other Asian countries.

