Korean botulinum toxin maker Medytox may likely suffer a delay in Chinese regulatory approval for Meditoxin (export name: Neuronox), industry sources said.
The Chinese regulatory agency suspended the approval review for the Korean product on May 24 and June 3, in addition to the similar move taken on March 13. Several suspension orders were unusual, and the Chinese agency seemingly aimed to demand additional data for Neuronox, sources said.
Medytox will likely have to wait for longer to obtain the nod in China due to the additional review process, they said.
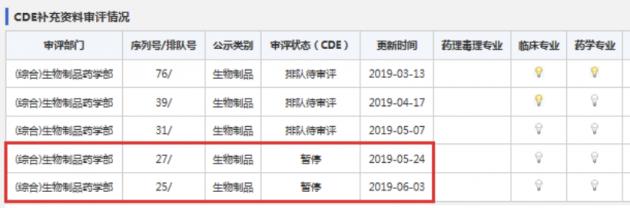
According to Yaozh.com, a Chinese online site for searching drug approval progress, the Center for Drug Evaluation (CDE) under the National Medical Products Administration (NMPA), formerly China Food and Drug Administration, ordered a suspension of review on Medytox’ Neuronox on May 24 and June 3.
Experts in Chinese drug approval said the Chinese authorities might have delayed the review because they had to convene a meeting of professionals for drug approval, among other possible reasons.
“To open an expert meeting to decide whether a drug should be allowed, the regulator might have stopped the review temporarily considering the time for the experts to gather. Once they are ready, they can resume the assessment,” an official at a consulting firm that helps to obtain Chinese sanitation certification. If it was this case, the review could be continuing, as Medytox said.
The company said on Wednesday in a statement, “Meditoxin approval in China is proceeding by the procedure, and the final document review is underway.”
However, the suspension of review might have signaled that the regulator needed additional data.
An official at a biotech firm’s approval team said the review suspension could occur when the Chinese authorities need detailed data or have to conduct an additional test. “As the regulator would need time to receive an answer from the company, it could temporarily discontinue the review process,” he said.
Industry watchers said a series of drug suspension orders were uncommon in China. They suspected that the Chinese regulator could be paying close attention to allegations about poor quality control at Medytox.
“Rumors are circulating that the Chinese drug review team not only requested Medytox to submit additional data but internally investigating into the company’s quality control issues,” said a Korean official who works for Korean drug approval in China. On this scenario, it is highly likely that additional data submission could delay the review process.
According to the CDE’s homepage, the agency had been evaluating additional data for Meditoxin since the suspension of review on March 13. As it normally takes a year to win sales approval, the market release of Meditoxin is expected to take more time.
“As we said in a statement on our homepage, it is not true that we received a notice of review suspension,” Medytox said. “The final review process is underway, and the company is waiting for results after submitting all the necessary data. We cannot confirm whether we will receive a request for additional data in the future.”

