The Korea Institute of Science and Technology (KIST, Director Lee Byung-kwon) has succeeded in developing the technology for evaluating the toxicity of anticancer drugs using small caenorhabditis elegans, or roundworms.
These nematodes are a 1-millimeter transparent bug that resides in soil and consists of over 900 somatic cells, 300 neurons, and 20,000 genes, KIST said in a press release Monday. As about 40 percent of the genes of small nematodes are preserved in humans, biological mechanisms such as cell death and aging can be applied, it added.
Sidney Brenner, who won the 2002 Nobel Prize in physiology and medicine, introduced the nematode into a biological laboratory, drawing enormous intellectual outcomes on life phenomena in modern genetics, embryology, and neurobiology in particular.
In the bio-industry, which develops pharmaceuticals and cosmetics, the toxicity evaluation process is necessary to ensure the safety of the product. Such assessments involve sacrifices of mammals such as rats, rabbits, and dogs, therefore raising issues of ethical issues and economics in animal testing.
Also, the risk of environmentally harmful substances such as fine dust, environmental hormones, heavy metals, pesticide residues, synthetic chemicals, and green algae toxins has risen so that the toxicity evaluation for estimating the risk of such environmentally harmful substances increased.
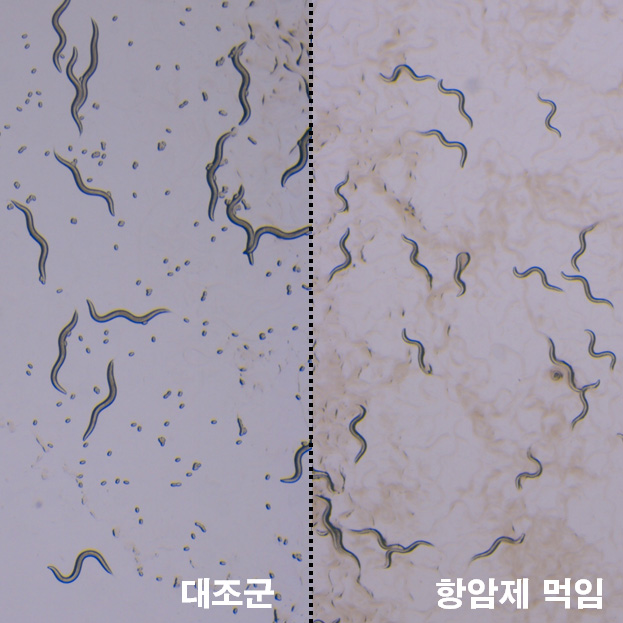
KIST researchers then chose nematodes as experimental animals to evaluate the toxicity of anticancer drugs on behalf of mammals, according to the press release. By feeding the anticancer agent to nematodes instead of an experimental mouse, observation of any problems in the behavior, the growth or the number of eggs produced by the insect is more affected, it added.
Compared with the efficacy and toxicity assessment process for the development of a new anticancer drug, researchers agreed that about 100 mice would have sacrificed for more than one month of research during the previous toxicity evaluation. Toxicological evaluation using the conventional method is based on mouse weight change, histopathological analysis, and blood test, while the worm test performed by variations is the size of worms, the number of eggs, hatching rate of eggs.
"Nematodes, although it is a worm, has digestive organs, neural organs and genes similar to humans, so in the future, not only will it be possible to evaluate the toxicity of anticancer drugs but also to elucidate the efficacy of various medicines and how they work can be used," said Kang Kyung-soo, a KIST fellow.
Researchers are currently conducting a toxicological evaluation test on a variety of chemicals, including cancer candidate agents and various environmentally harmful substances, using the nematode assessment method.
The researchers also plan to utilize the worms to verify efficacy and side effects in the development of new medicines and cosmetics, such as the development of health functional foods that improve intestinal health from natural products.
By minimizing the sacrifice of mammals, the ethics of animal research can be preserved, and the efficiency of research focusing mammalian experiments on essential parts can enhance.

