Korea’s pharmaceutical and biotechnology industries have cited the government’s support for investment in research and development and the expansion of tax benefits as the most necessary policies, a survey shows.
Korea Pharmaceutical and Bio-Pharma Manufacturers Association (KPBMA)한국제약바이오협회(Chairman Won Hee-mok원희목) conducted the survey.
To find out their levels of satisfaction with the status of domestic pharmaceutical and biomedical industry, it asked 28 questions in five areas, on a scale of five points. And to find out demands from the pharmaceutical industry about the government’s supportive measures for the sector, it also asked 51 questions in five areas.
According to the results, these industries showed low satisfaction with tax benefit and information provision policies. The pollsters gauged the satisfaction level in five areas – science-technology and infrastructure, the current state of affairs and environment for clinical research, regulatory system, market entry and financing, and the protection of intellectual property rights.
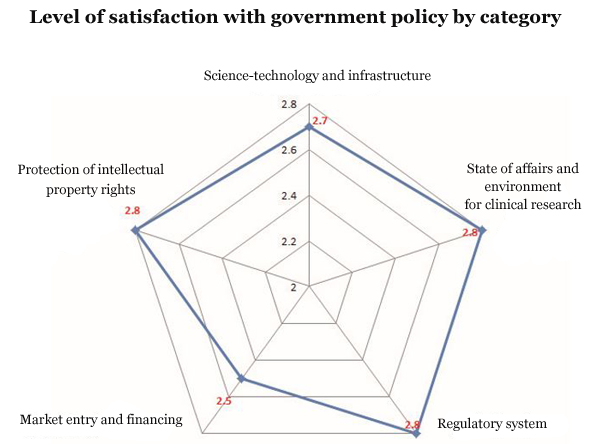
Respondents gave three points or less on average to all these categories
In the market entry and financing category, in particular, tax benefits related to biomedicines (such as R&D, infra, and clinical trials) and information provision on biomedicines for patients and the public (such as ads, academic journals, information on medical workers) received the lowest points of 2.3 on average.
“Tax benefit is a top priority policy for the industries and also marks the beginning of a virtuous circle to reinvest in R&D,” the report said. “The low satisfaction level indicates the government needs to shift its policy toward concentrating R&D investment support on a selected few.”
Reflecting their low satisfaction with the government’s support policy, the industries expected the new government would implement various policies to aid the sector. In demand survey in particular, the industry showed high demands for regulatory control, market entry and financing.
The poll surveyed the demand levels in five categories, too -- R&D, infrastructure, regulatory control, creating bio ecosystem, and market entry and financing. The average points of these categories were higher than 3.5 points. Receiving highest mark was the regulatory control-market entry-financing category, and the query asking about tax benefit for biomedicine R&D and infrastructure investment got the highest satisfaction level of 4.2 points on average.
“Because biomedicines require a massive amount of investment in infrastructure, ranging from clinical trials to specialized research workforce, high-tech equipment, and production facilities at the early development stage, the government needs to support and invest in these fields,” the association said.
It went on to say, “The government has to include clinical trials on incrementally modified drugs and biosimilar in its designation as new growth engine driving technologies, and has to expand tax breaks, including those for labor costs and R&D expenses paid to clinical testing agencies.”

