About three years have passed since the Korean government started to grant selective health insurance coverage for next-generation sequencing (NGS)-based gene panel tests for 10 most common types of cancer in March 2017.

From May last year, the scope of reimbursement expanded to all types of solid cancer, and Korean hospitals have accumulated more than 7,000 cases of NGS data per year. However, no national database is available for integrating relevant NGS data with clinical data, drug prescriptions, and therapeutic outcomes.
In a recent interview with Korea Biomedical Review, Korean Cancer Study Group President Kang Jin-hyoung said NGS data obtained through the national health insurance program belongs to Korean people who are the subscribers of the health insurance, although hospitals and other interested parties might have different opinions.
“The government, on behalf of the public, should manage NGS data,” said Kang, who is also a professor at the Oncology Department of the Catholic University of Korea School of Medicine.
He took an example of Japan, which was two years behind Korea to allow insurance benefits for NGS testing.
By learning lessons from Korea’s trials and errors, the Japanese government was making NGS data a national asset to use it for research and development, patient care, drug approval, and insurance, Kang emphasized.
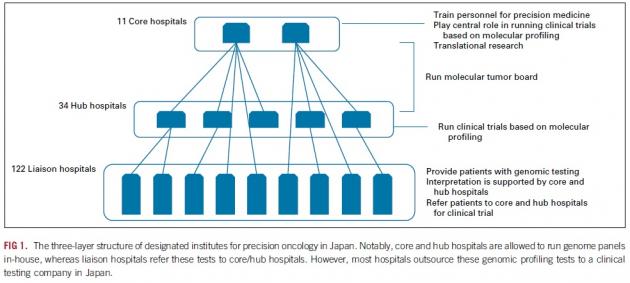
According to a paper, “Precision Oncology and the Universal Health Coverage System in Japan” published in the American Society of Clinical Oncology on Dec. 11, Japan established a center to integrate the network of hospitals that had already conducted NGS testing before the reimbursement and to collect and oversee genomic profiling of patients.
Japan designated more than 170 medical institutions for NGS testing. The hospitals are divided into three-layered groups – 11 core hospitals that nurture talents for precision medicine and play a central role in clinical trials, 34 hub hospitals that run trials, and 122 liaison hospitals that provide NGS testing for patients.
Also, Japan allowed only two NGS genetic panel testing for reimbursement among numerous NGS gene panels. One is “FoundationOne CDx Cancer Genomic Profile” by Foundation Medicine, recently acquired by Roche, and the other is the “OncoGuide NCC Oncopanel System” developed by Japan’s National Cancer Center.
Japan’s NCC established the Center for Cancer Genomics and Advanced Therapeutics (C-CAT), which will serve as a central data repository to collect genomic information and clinical characteristics of patients who received NGS testing and to support information that can be linked to clinical trials.
Consequently, Japan accumulated data of 13,000 Asian patients a year and secured a strong national asset through C-CAT.
“In Korea, NGS data is stored in each hospital’s pathology department. We need to combine the genomic data and clinical information accumulated in many hospitals,” Kang said. “Genomic data is left unused, it becomes worthless. We need to use this data to diagnose and treat cancer and monitor the performance."
However, interested parties – such as the National Health Insurance Services, the Health Insurance Review and Assessment Service, the Korea Centers for Disease Control and Prevention, and the National Cancer Center – could regard this data as their respective assets, he noted.
“This is why we need some forceful systems. Based on the assumption that subscribers of the national health insurance program can benefit from the health data the government collected through their payment, we need to make it into law or regulation,” Kang proposed.

