ImmuneMed said the company and Seoul National University Hospital (SNUH) have won the government’s approval for Virus Suppressing Factor (VSF) as the treatment for new coronavirus patients, and are administering it on some subjects.
The Ministry of Food and Drug Safety gave the go-ahead to SNUH’s request for using ImmuneMed’s VSF on COVID-19 patients on Feb 21.
The two partners are planning to inject VSF four times -- first, third, seventh and 14th day -- from the initial administration. The university, company, and the ministry will jointly monitor the drug’s effects and side-effects after administering it.
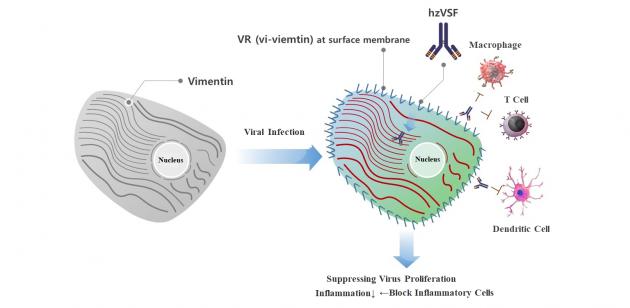
The treatment is known as ‘HzVSFv13,' an injection-type of the company’s VSF. ImmuneMed had conducted phase 1 clinical trials on healthy men since 2018.
In the clinical trial conducted at SNUH, the researchers administered intravenous doses and evaluated their safety, drug-tolerance, and pharmacokinetic properties. The final result report of the clinical trial is expected to be released in April
"HzVSFv13 shot is one of the first treatments to receive approval from the ministry and was applied to COVID-19 patients. It will help recover the pneumonia patients with more than moderate symptoms," ImmuneMed CEO Kim Yoon-won said. "We hope we will be able to give VSF to such COVID-19 free of charge and shorten their treatment time and lower fatalities, by cooperating with hospitals."
ImmuneMed also plans to develop VSF-based treatments for chronic hepatitis B, influenza, and some inveterate skin diseases. The company drew the industry’s attention when it cured 50 percent of the woodchucks with hepatitis B in animal experiments last year.
The company said it is also waiting to conduct phase 2 clinical trials to win hepatitis B indication.

