Sanofi Aventis has launched its hemophilia B treatment Alprolix (Ingredient: Eftrenonacog alfa, Fc Fusion protein), a blood coagulation factor 9 with an extended half-life in Korea on Thursday.
Alprolix was approved as conventional therapy for preventing hemophilia B patients from bleeding, managing perioperative periods, and reducing bleeding frequency.
One injection of 50 international units per kilogram a week or 100 international units per kilogram in 10 to 14 days interval can prevent the patient from bleeding.
The blood coagulation factor 9, extended by the fragment crystallizable fusion (Fc fusion) technology, was allowed as the therapy. The treatment has reduced the number of previous intravenous injections, from about 100 to 50.
Patients can expect treatment convenience and improvement in life quality, and pediatric patients who have difficulty in finding veins can also benefit from the drug.
The Fc Fusion technology combines the blood coagulation factor 9 with Fc regions of immunoglobulin G1 (IgG1). The Fc region of Alprolix extends the half-life by recycling without being dismantled by lysosome and binds to the neonatal Fc receptor (FcRn) of the human body. Alprolix's terminal half-life is 82.1 hours, which is 2.4 times longer than the treatments with a normal half-life of 33.8 hours.
Alprolix confirmed the efficacy and safety profile with B-LONG clinical studies.
The group with weekly administration of prophylaxis showed a median annualized bleeding rate of 3.0, and the interval-adjusted prophylaxis group showed a rate of 1.4. These two groups showed a lower value than the episodic treatment group of 17.7.
The median annualized spontaneous joint bleeding rate of the episodic treatment group was found to be 0.0.
Among the 636 bleeding episodes, the Alprolix administration controlled 90.4 percent of the participants with a single injection, and 97.3 percent with one or two.
Inhibitor was not found, and anaphylaxis has not been reported, either. Also, the inhibitor was not detected in the four years of study, called B-YOND.
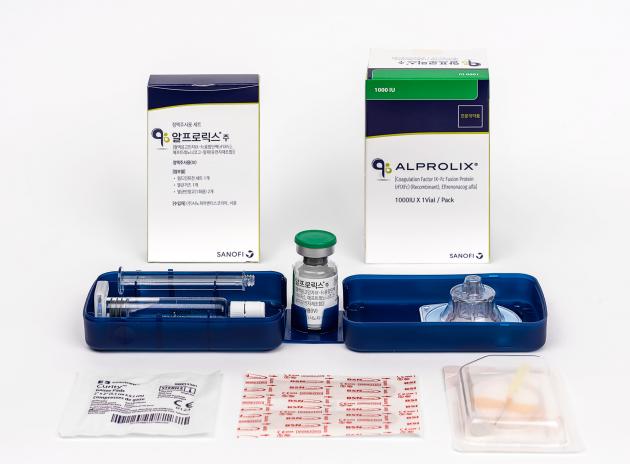
Sanofi Aventis introduced a hard case package that can be stored safe and easy to use. The reusable package has Alprolix vial, vial adapter, prefilled syringe, and plinger stick for the convenience. The case is also designed to fixate components with a risk of damage, such as the vial.
"With the launch of Alprolix, we can provide treatments with an extended half-life for hemophilia B patients who have had relatively narrow treatment options," said Park Hee-kyung, CEO of Sanofi Genzyme, the business unit of Sanofi Aventis Specialty Care.
Sanofi will try its best to contribute to the hemophilia community by a pipeline of hemophilia treatment with Alprolix and soon-to-be-released Eloctate, hemophilia A treatment with an extended half-life, he added.
The one-time injection, according to reimbursement standards set by Health Insurance Review and Assessment, is 30 international units per kilogram for adults and 41 for children. Moderate patients can receive up to 50 international units per kilogram depending on the physicians' decision, which can increase to 70 for pediatric patients
The launching products' volumes are 250, 1000, 2000 international units, and the company is planning to increase them up to 500 and 3000 international units in the future.

