Pharmaceutical companies have been increasingly developing drugs for rare diseases, with these drugs accounting for 32 percent of the forecasted $1.06- trillion prescription drug sales by 2022, according to a recent report by EvaluatePharma.
The so-called orphan drugs target a small population of patients who have a rare disease. In the United States, thousands of rare diseases affect fewer than 200,000 people, with the FDA estimating 85 to 90 percent to be serious or life-threatening. Despite the high mortality rate, most patients with the rare disease have struggled without cures, however.
“People who suffer from rare diseases are too often faced with no, or limited, treatment options, and what treatment options they have may be quite expensive due in part to significant costs of developing therapies for smaller populations,” said FDA Commissioner Scott Gottlieb.
In the past, pharmaceutical companies had focused on developing blockbuster drugs for large populations. They have avoided developing orphan drugs due to high research and development costs and low profit margins.
However, those days are long gone, according to the Food and Drug Administration, which stated the number of orphan drug designation requests has doubled since 2012 in the FDA’s Orphan Drug Modernization Plan announced last Tuesday.
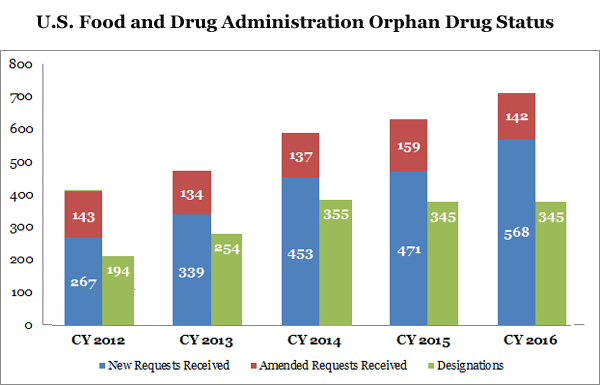
The reason for the upward trend is multifaceted, with companies finding the sector of rare disease development having less competition while also having plushy regulatory grounds that expedite the review process and lower cost. Pharmaceutical companies are now racing to create the biggest breakthroughs in rare diseases and to double down on drug patents.
Much of the growth is expected to come from the industry’s trending sectors such as anticancer drugs like Opdivo and Keytrudra, and high-risk assets such as Alzheimer’s, according to the report.
The FDA is also ramping up efforts to keep up with the growing market for rare-disease drugs, announcing its plan to handle orphan designation request backlog within 90 days by mid-September. The agency has established a Backlog SWAT team “comprised of senior, experienced reviewers with significant expertise in orphan drug designation,” along with other measures. The FDA will also “ensure continued timely response to all new requests for designation with firm deadlines.”
The federal agency’s renewed commitment is a continuation of its efforts to promote the sector, establishing the Orphan Drug Act in 1983. The Orphan Drug Designation Program provides orphan status to “drugs and biologics that are defined as those intended for safe and effective treatment, diagnosis, or prevention of rare disease,” according to the FDA.
The agency announced that it currently has around 200 pending orphan drug designation requests and 568 new applications.

