Korea has become one of the first countries to warn the public of the depressive side-effects of finasteride, a hair loss medication, according to the Post-Finasteride Syndrome Foundation.
The 5-alpha-reductase inhibitor drug, commonly known as Propecia, is an anti-balding drug used for hair loss and prostate enlargement. Some men who have taken finasteride have reported symptoms of depression, suicidal ideation, and anxiety, in addition to previously reported symptoms of loss of libido and erectile dysfunction.
The World Health Organization (WHO) published the depressive effects of the drug two years ago with extensive research to show finasteride may cause depression and suicidal thoughts.
According to the Post-Finasteride Foundation, the World Health Organization Programme for International Drug Monitoring found finasteride-induced suicidal and self-injurious behavior rose 33 percent from the third to fourth quarter of 2015.
Within the category of psychiatric disorders, suicidal ideation rose 47.2 percent, suicide attempts 28.5 percent, and completed suicides 7.2 percent, indicating an increase from 55 suicide cases to 59. Drug users reported a total of 2,991 adverse drug reactions (ADR) that fell under psychiatric disorders, according to the WHO.
As for the type of psychiatric disorder, libido decrease had the most cases with 1,248 reports, followed by depression (1,160 cases), anxiety (975), insomnia (358), suicidal ideation (199), and panic attacks (130), among others.
Following the WHO publication, the New Zealand Medicines and Medical Devices Safety Authority (MEDSAFE) was the first institution to publish warnings of these conditions in March 2016, followed by the United Kingdom’s Ministry of Healthcare products Regulatory Agency (MHRA) in May 2017. Korea’s Ministry of Food and Drug Safety announced changes to finasteride warning labels Tuesday, becoming the third country to issue a public warning.

“If you look at all the nations on earth, some 195 have yet to warn their citizens of the full risks posed by finasteride,” said Philip Roberts of the Post-Finasteride Syndrome Foundation in an email interview with Korea Biomedical Review.
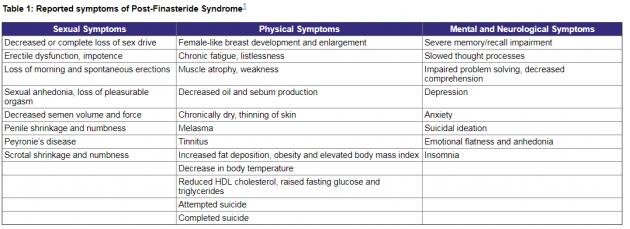
The syndrome, which can occur in men who have taken finasteride, include sexual, physical, mental, and neurological symptoms that persist after the patient has stopped taking the drug.
“It seems safe to say at this point that finasteride is the source of persistent adverse reactions only in a subset of men who take the drug,” Roberts said. “The question is how big that subset is.”
Dr. Landon W. Trost, head of andrology at the Mayo Clinic, estimates that 5 percent of all men who take finasteride experience erectile dysfunction, stressing the importance of raising awareness of PFS.
“Of the nearly 1,000 PFS patients we have spoken to around the world over the past five years, virtually every single one has said if they had been aware of all the potential side effects, they would have never taken it,” Roberts added.
The foundation, along with the three government institutions that have published reports, emphasizes the importance of being educated about potential side effects.
“One of the most common problems PFS patients experience is being told by one or more doctors that the condition does not exist, that it’s all psychosomatic. That often thrusts the patients into deeper despair,” Roberts said. “We urge all doctors, mainly dermatologists, and urologists, to carefully review all the clinical studies of the PFS Foundation and also urge any men suffering from PFS to participate in our Patient Support program, which connects PFS patients with one another for mutual support and sharing of coping strategies.”

