The Korean Pharmaceutical Association said Wednesday that some of Sanofi Aventis' Rodogyl 125mg products, a susceptible anaerobic bacteria infection treatment, had defects in their press through pack (PTP) packaging, calling for caution in distributing them.
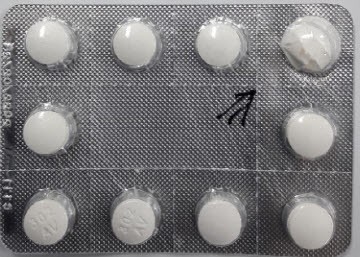
The association’s move followed its request in December last year for Sanofi’s correctional measure, after discovering that some batches of the Rodogyl 125mg had crushed tablets inside the packing.
The product in question uses C111 as its manufacturing code with an expiration date of Feb. 28, 2022.
Afterward, Sanofi acknowledged that there was a problem with the process in their packaging line and promised to voluntarily retrieve the commercially available products before adjusting their manufacturing process. Despite the company's promise, the association said it could still find the defect batches with the corresponding manufacturing number at some of its member pharmacies.
Accordingly, the association requested Sanofi to identify and quickly recover the defect Rodogyl batch from pharmacies, while advising pharmacies to return all defect products to their wholesalers.
"Some pharmaceutical companies are responding lukewarmly to our request, even though medicines directly related to people's lives are being supplied in poor condition," the association said. "We strongly request that Sanofi recover the drugs as soon as possible."
KPA will continue to take the lead in identifying the causes of the distribution of defective drugs and prepare measures to prevent a recurrence, the association added.
In response to the association's remarks, a Sanofi Korea spokesperson said that the company is aware of the situation.
"The company has confirmed that the defect drugs occurred in the packaging line process for products using the manufacturing number C111," the spokesperson told Korea Biomedical Review. "As a result of the investigation of complaints, we confirmed that the broken tablets were positioned vertically inside the packaging and not horizontally."
The defect is only limited to manufacturing number C111, and the company has confirmed that defects were not shown in any of the other manufacturing numbers, she added.
The spokesperson stressed that while the defect drug does not have any problems in terms of safety and efficacy, the company decided to retrieve the product voluntarily in December last year, considering the inconvenience it may cause for patients.
"The company is trying to complete the voluntary recovery as quickly as possible," she said. "If any pharmacies still have the drug, please return it to the wholesaler immediately."

