With growing anticipation for the market release of Covid-19 vaccines, people are worrying that only a small number of rich countries could grab a majority of the Covid-19 vaccine supply. Such concern has become reality.
A report on Monday showed that the European Union and five countries, accounting for only 13 percent of the world’s population, have secured about half of the planned production of Covid-19 vaccines.
Nature published the report, citing data from Airfinity, a life sciences market analytics firm in the U.K.
So far, AstraZeneca, Pfizer, and Moderna have come closest to the commercialization of the Covid-19 vaccine. The three companies said they could manufacture 5.3 billion doses in total by 2021 which could be administered to between 2.6 billion and 3.1 billion people, depending on whether AstraZeneca’s vaccine is given in two doses or one and a half.
According to Nature, 27 states of the EU and five rich countries including Canada and the U.S. have pre-ordered half of the vaccines available for production. The 31 nations account for only around 13 percent of the global population.
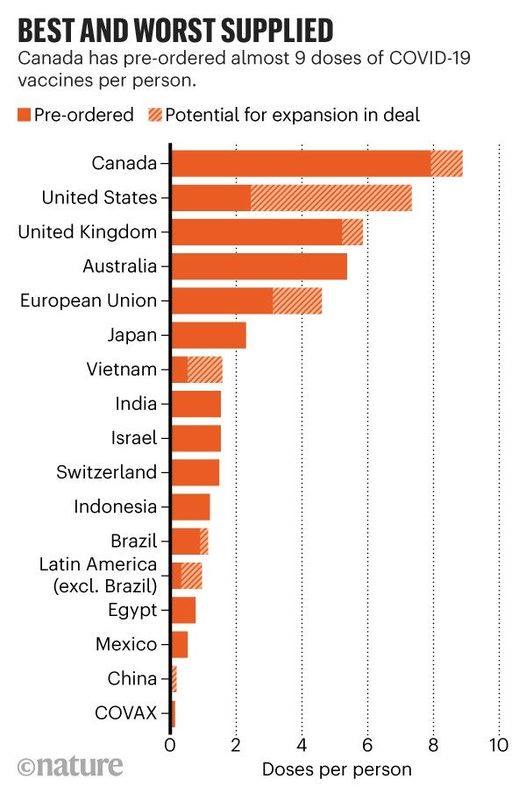
Canada ranked first in securing Covid-19 vaccines, preparing nine vaccine doses per capital.
The U.S. came in second with seven doses per capita, followed by the U.K. and Australia with five doses, respectively. Japan preordered two Covid-19 vaccine doses per capita.
Many countries are trying to obtain Covid-19 vaccines from COVAX, a joint fund for the fair distribution of Covid-19 vaccines around the world.
Gavi, a Geneva-based organization to support vaccines for low-income countries, the World Health Organization (WHO), and the Coalition for Epidemic Preparedness Innovations (CEPI) are running COVAX. More than 189 countries have joined COVAX so far.
Korea plans to rely on COVAX for Covid-19 vaccines for 10 million people. However, the country has yet to preorder a Covid-19 vaccine.
The Korean health authorities have nearly wrapped up discussions with individual pharmaceutical firms over Covid-19 vaccine purchase deals, said Jeong Eun-kyeong, commissioner of the Korea Disease Control and Prevention Agency (KCDA), in a briefing on Monday. “After consulting with the budget authorities, we will release the discussion results by next week or the early week after the next week,” she said.
As the government has to preorder unauthorized vaccines, it needs more information on their safety, she noted.
“We plan to secure quantities according to each manufacturing method -- such as virus carrier vaccine, mRNA vaccine, and synthetic antigen vaccine. Then, in real vaccination situations, we will plan the purchase and vaccination making sure those who need the vaccine the most get it first,” she said.

