
The Covid-19 vaccine, “mRNA-1273,” jointly developed by Moderna and the U.S. National Allergy and Infectious Disease Research Institute (NIAID), can maintain immunogenicity for 90 days after the last injection, Moderna said Thursday.
Researchers at NIAID inoculated 34 healthy adult participants twice with mRNA-1273 100-μg. The team divided them into three age groups -- 18-55, 56-70, and 71 or older.
Despite a slight decrease over time, the results showed that all participants maintained high binding and neutralizing antibodies after three months of the second inoculation.
The Geometric mean titer (GMT) assessed by the enzyme-linked immunosorbent assay showed 23,5228 in the 18-55 age group, 151,761 in the 56-70 age group, and 157,956 in the age group over 71, respectively.
Besides, in the data showed on day 119, all participants had neutralizing antibodies of the serum in the test using pseudovirus. The combined and neutralized anti-drug immunogenicity were above 41, the control groups' median scores recovering from the coronavirus. There were no side effects after 57 days concerning the vaccines, they said.
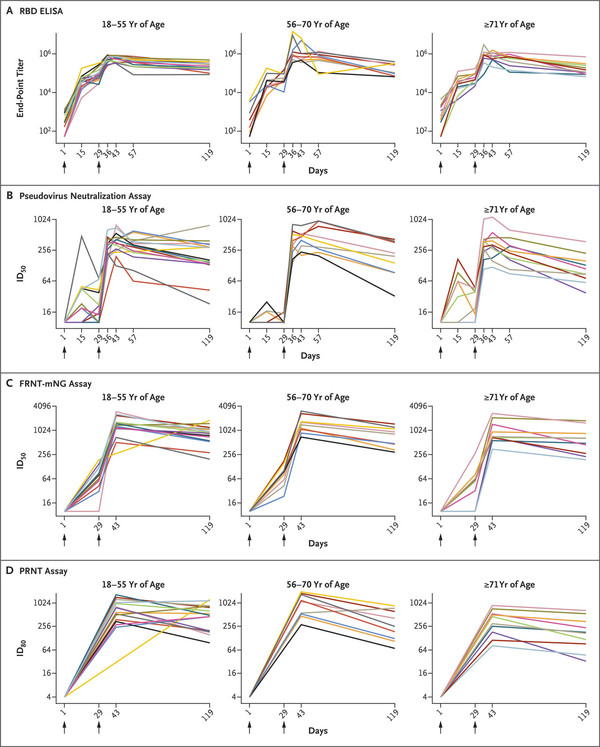
According to the team, the results demonstrated mRNA-1273 could provide continuous humoral immunity, despite a slight reduction in binding and neutral antibody potency. The finding will support the ongoing phase 3 trials, which marked a 94.5 percent efficacy rate.
“We will continue to evaluate the long-term response of the vaccines, conducting follow-up analysis for 13 months to test the safety and immunity of the vaccine."
The results were published in the Correspondence section of the New England Journal of Medicine (NEJM). While the previous data contained the results of 57 days after injection of the vaccines, the current one presents data of 90 days after the second vaccination.

