Cellid is speeding up a phase-2b/3 trial of AdCLD-CoV19, a Covid-19 vaccine candidate, and getting ready for mass production, raising hopes that a homegrown Covid-19 vaccine will arrive soon.
“We’ve already produced vaccines for a phase-3 trial at the Animal Cell Culture Substantiation Center in Andong. We plan to develop a manufacturing process for additional mass production jointly with the center,” an official at Cellid said Thursday.
AdCLD-CoV19 is a viral vector vaccine manufactured by putting the spike gene of the Covid-19 virus into an adenovirus template, a type of cold virus. It is the same type developed by AstraZeneca and Janssen.
Cellid won the regulatory nod for the phase-1/2a trial of the vaccine candidate on Dec. 4. For mass production of the vaccine, the company formed a consortium comprised of LG Chem, Jeollanam-do Biopharmaceutical Research Center (KBRC), and the Animal Cell Culture Substantiation Center in Andong, North Gyeongsang Province.
However, LG Chem, which signed an MOU with Cellid for joint research and mass production of a Covid-19 vaccine last year, decided not to participate in the consortium.
An official at Cellid said the MOU did not lead to a final deal with LG Chem. “Although the two companies have conducted research together, LG Chem cannot secure a separate GMP line exclusive for AdCLD-CoV19 production,” he said.
An official at LG Chem declined to comment.
For this reason, Cellid is looking for a second contract manufacturing organization (CMO) for mass production of AdCLD-CoV19, after the Animal Cell Culture Substantiation Center.
“We’re contacting several CMOs,” an official at Cellid said.
Cellid pushes for global vaccine trials for patient recruitment
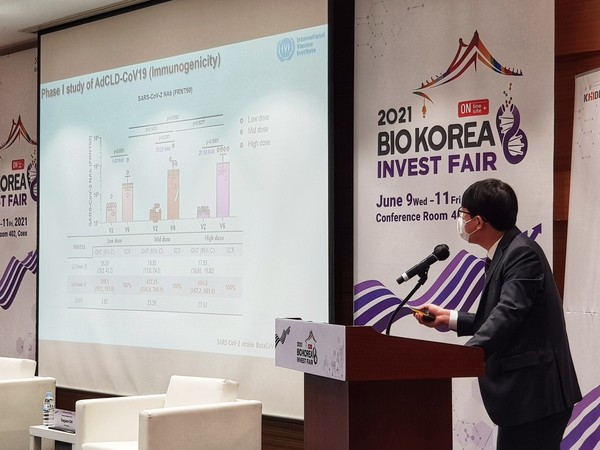
Cellid released the interim results of the phase-1 trial of the Covid-19 vaccine candidate at the Invest Fair during Bio Korea 2021 at COEX, Seoul, Wednesday. The company said it partially confirmed AdCLD-CoV19’s safety and immunogenicity.
Cellid has completed patient recruitment for a phase-2a study of the vaccine candidate and the vaccine administration. The company is collecting samples before analyzing the results.
the phase-1 study, Cellid administered a single low-dose, medium-dose, and high-dose vaccine to 10 trial participants each and observed geometric mean titer (GMT) of binding and neutralizing antibodies at the second week and the fourth week. With the final phase-1 results, the company plans to derive T-cell response data.
Shin Kwang-soo, a research scientist at Cellid who presented the data at Bio Korea 2021, said, “As the dose increased, the number of adverse events tended to rise, but no serious adverse events of grade 3 or higher occurred.” The most common adverse reactions included inoculation site pain, which is a common adverse reaction after vaccination.
“AdCLD-CoV19 did not cause adverse reactions that were more serious than those of other Covid-19 vaccines,” he added.
According to Cellid, a significant level of binding antibody was induced in all 30 participants. In addition, antibodies that bind to the receptor-binding domain (RBD) protein were also converted to antibody-positive in all participants.
In the case of neutralizing antibodies, all participants showed significant antibody titer levels compared to baseline.
However, the company has to tackle an issue -- the antibody titer and dose increase were not proportional at the fourth week of injection.
“At the fourth week of vaccination, there was no significant difference in antibody titers between the medium and the high dose groups. This is probably due to the small number of participants in the phase-1 trial where each treatment group had 10 participants,” Shin said. “We need more verification. After we conduct a phase-2a study, we will be able to assess more accurately.”
Cellid hopes to begin a phase-2b trial in July and a phase-3 trial in September. It also considers conducting the trials overseas.
“Based on the phase-2a results, we will start a non-inferiority study comparing the vaccine candidate with existing authorized vaccines,” Shin said. “It is expected to enroll patients in Korea only, so we are aggressively pushing for a global trial.”

