The global regenerative medicine industry is growing rapidly, and the Asia-Pacific region is a major center of growth for the sector driven primarily by the recently added new Korean and Taiwanese clinical trials, a report said.
There are 1,320 industry-sponsored regenerative medicine and advanced therapies trials going on worldwide, an increase of 100 since the end of 2020, mainly due to trials registered by Korea and Taiwan, according to the Alliance for Regenerative Medicine (ARM) report by released by KoreaBio.
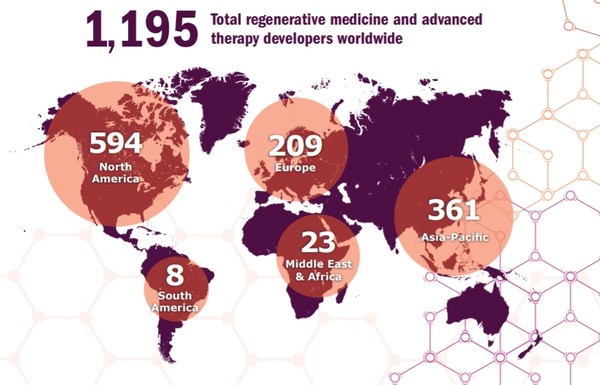
Currently, 1,195 companies are running 1,320 clinical trials of regenerative medicine, of which 158 are phase 3 trials, it said.
The investment in the regenerative medicine industry continued to increase to $14.1 billion in the first half of 2021, up 35 percent from a year ago. Regenerative medicine is a branch of medicine that restores the functions of human cells, tissues, and organs, emerging as innovative treatment alternatives for rare and intractable diseases.
Among the 1,320 trials, 294 are for gene therapy, 501 for cell-based immuno-oncology, 504 for cell therapy, and 21 for tissue engineering.
By disease, there are 641 tumor-related clinical trials, followed by 94 cases of the central nervous system, 85 cases of infectious diseases, and 76 cases of rare genetic disorders.
The report said that various discussions are now being made in advanced countries to establish a financial support system to reduce patient burden.
Considering the specificity of regenerative medicine using living cells, Europe has a separate safety management system and guarantees and manages patients' access to regenerative medicine treatment.
As the implementation plan for advanced regenerative medicines and biopharmaceuticals has been promoted in Korea from last year, clinical trials of regenerative medicine are likely to become more active from the second half of the year.
Since the government introduced the Advanced Regenerative Bio Act in August 2020, interest in biopharmaceuticals with the latest technologies, such as stem cell therapy and gene therapy, has been growing.
The government plans to complete an institutional framework of the safety management system for clinical trials of advanced regenerative medicine and biopharmaceuticals, a professional review and management system, and a long-term follow-up investigation.
The Ministry of Health and Welfare will invest 79 billion won ($67.3 million) this year to support the development of advanced medical technologies, clinical trials expenses for regenerative medicine, and support for research-oriented medical institutions.
Health authorities plan to expand the target for a designated regenerative medical institution to general hospitals and actively support research on innovative treatments for rare and incurable diseases by funding clinical trials.
To conduct clinical trials related to advanced regenerative medicine, a hospital must win the designation as an advanced regenerative medical institution and get approval for its research plan.
The ministry aims to reduce the time required for approval by applying for designation and the application for the research plan.
"Next-generation CAR-T therapies will combine with other technologies to enhance potency and targeting, and genetic modification will allow scientists to turn therapies on or off, while gene editing platforms could help to create more potent cells," said Bruce Levine, an ARM board director. "We will equip the human body to fight cancer, and the full potential of cells as medicines will bring extraordinary benefits to patients."

