Samyang Biopharmaceuticals has developed Lenalidomide, a therapeutic agent for multiple myeloma, company officials said Friday.
Lenalidomide tablets are mainly for elderly patients by solving the discomfort of the capsules which stick to the esophageal mucosa in the mouth.
Samyang’s Lenalidomide is a generic of Celgene Korea세엘진코리아 Revlimid capsules with its volume one-third (based on 25mg) of the capsule products.
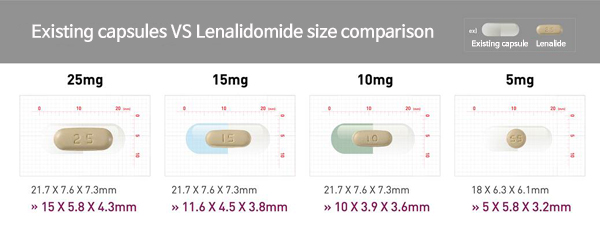
Samyang삼양바이오팜 (CEO: Um Tae-woong엄태웅) plans to diversify its treatment options by selling 20, 7.5, and 2.5mg doses in addition to the four types of drugs (25, 15, 10, 5mg)
Samyang is also promoting its fields of medical devices that utilize anti-cancer medicines and biodegradable materials based on DDS platform technology.
"We are currently applying for an insurance policy and will release it after the patent expiration date of Lenalidomide on Oct. 27," a company official said. “It is possible to take exactly the dose needed for each dose cycle with subdivided dose.”
Meanwhile, Samyang has released 2.5mg of Bortezomib, a generic drug of Velcade, on Aug. 1. Bortezomib is the first treatment for multiple myeloma treatment.
The price of 2.5 mg of Bortezomib will likely reduce the financial burdens of patients as it downed its price to the lowest level among similar products in the domestic market, they said.

