Pirfenidone, a treatment for idiopathic pulmonary fibrosis with its original drug’s patent expired, showed a remarkable therapeutic effect in heart failure with preserved ejection fraction (HFpEF), a study showed.
The study drew much attention because researchers have failed to develop an HFpEF treatment so far.
The outcomes of the investigator-initiated PIROUETTE trial were presented at the Late-Breaking Clinical Trials during the meeting of the American College of Cardiology (ACC 2021) on Monday. Clinical scientists and cardiologists led the study at the U.K.’s National Institute for Health Research (NIHR).
The PIROUETTE study is a randomized, double-blind, placebo-controlled phase-2 clinical trial that compared the anti-fibrotic drug pirfenidone to placebo in patients with myocardial fibrosis, one of the major causes of HFpEF.
Christopher Miller, a Ph.D. clinical scientist at the University of Manchester, who presented the study, said HFpEF, accounting for about half of heart failure diseases and having a high mortality rate, is a disease with the most unmet needs in cardiovascular treatment area.
Although HFpEF has various pathological mechanisms, myocardial fibrosis is known to be associated with death and hospitalization for heart failure in HFpEF patients, he said.
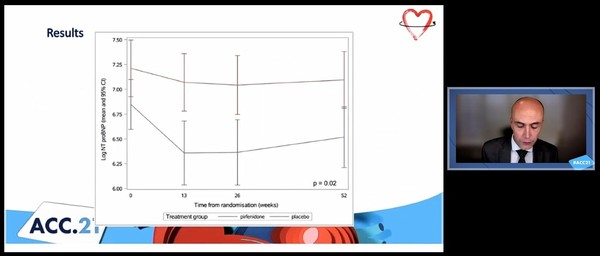
The research team evaluated myocardial fibrosis through cardiovascular magnetic resonance imaging (CMR) in patients with heart failure with a left ventricular ejection rate greater than 45 percent, NTproBNP greater than 300 pg/ml, and BMP greater than 100 pg/ml. Then, the researchers randomly assigned pirfenidone and placebo to patients with extracellular volume (ECV) greater than 27 percent and measured changes in myocardial fibrosis through CMR after 12 months.
The results showed that at 52 weeks, ECV declined 0.7 percent in the pirfenidone group and increased 0.5 percent in the placebo group. There was a 1.21 percentage point difference between the two groups. The difference was statistically significant even in sensitivity analysis and random analysis.
However, the two groups showed no difference in left ventricular relaxation function and ventricular and atrial function. Also, the six-minute walking distance has not been improved statistically meaningfully.
Serious adverse reactions occurred in 12 people (26 percent) in the pirfenidone group and 14 (30 percent) in the placebo group. There was no difference in heart-related adverse reactions between the two groups.
“Pirfenidone reduced myocardial fibrosis in HFpEF patients with myocardial fibrosis,” Miller said.
The results signaled that pirfenidone could provide clinical benefits for HFpEF patients, and to confirm this, additional research is needed to verify pirfenidone’s clinical efficacy and safety, he concluded.
Although observational studies showed that changes in ECV in patients with heart failure were associated with a 9 to 28 percent reduction in death and heart failure hospitalization, a prospective study is required, Miller went on to say.
He noted that there was no change in left ventricular relaxation function, and this might be controversial.
Roche is the developer of the original drug Esbriet using pirfenidone, but the substance patent has expired. Pirfenidone is available in generic drugs in Korea.
The government data showed that four local drugmakers are selling eight pirfenidone-containing products.

