MSD’s antidiabetic drug Steglatro (ingredient: ertugliflozin), a sodium-glucose cotransporter 2 (SGLT-2) inhibitor, demonstrated clinical benefits in renal functions, a study said.
The results enhance evidence that SGLT-2 inhibitors could be beneficial in kidney disease.
An exploratory analysis of the VERTIS-CV trial of Steglatro found that Steglatro reduced the risk of composite renal events by 34 percent, compared to placebo. The results were published in the March issue of Diabetologia, a journal of the European Association for the Study of Diabetes (EASD).
The VERTIS-CV study assessed the cardiovascular safety of Steglatro compared to placebo in 8,246 patients with type-2 diabetes with atherosclerotic cardiovascular disease from December 2013 to December 2019.
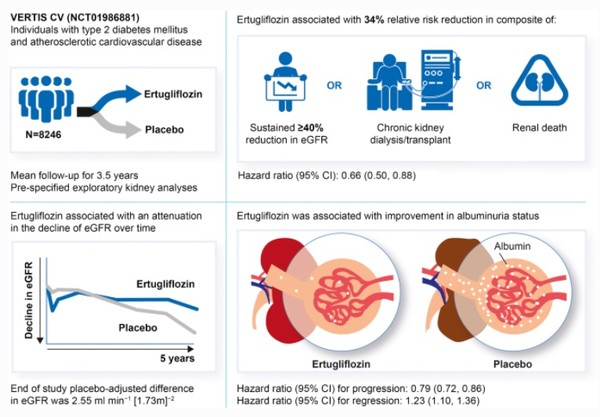
The secondary endpoint of the VERTIS-CV study comprised of doubling of serum creatinine from baseline, renal death, and renal dialysis/transplant. The exploratory analysis replaced doubling of serum creatinine from baseline with a sustained 40 percent decrease from baseline in estimated glomerular filtration rate (eGFR).
According to the analysis, the kidney composite outcome occurred in six per 1,000 people in the Steglatro group, 34 percent lower than nine per 1,000 in the placebo group.
The annual incidence of sustained 40 percent reduction from baseline in eGFR was 5.8 per 1,000 in the Steglatro group, 34 percent lower than 8.8 per 1,000 in the placebo group.
Also, at 60 months, the Steglatro group had a 16.2 percent lower urinary albumin/creatinine ratio (UACR) and a 2.6 mL/min/1.73m² higher eGRF than the placebo group.
In the sub-group analysis, Steglatro showed a consistent decrease in UACR and attenuation of eGFR decline. The treatment demonstrated a stronger effect in the macroalbuminuria and Kidney Disease: Improving Global Outcomes in Chronic Kidney Disease (KDIGO CKD) high/very high-risk subgroups.
In a sub-analysis of the VERTIS-CV study released in December, Steglatro reduced the risk of hospitalization due to heart failure. Its benefits in heart failure were consistent with those of existing SGLT-2 inhibitors.
The sub-analysis results showed that the risk of hospitalization due to initial heart failure was significantly lower in the Steglatro group (2.5 percent) than in the placebo group (3.6 percent). Steglatro also reduced the risk of death caused by heart failure-induced hospitalization and cardiovascular reasons.
The lowered risk of hospitalization due to initial heart failure with Steglatro was consistent in most sub-groups. The benefits were greater in patients with baseline eGFR less than 60mL/min/1.73m² or albuminuria, those taking diuretics, and patients taking loop diuretics.

