Two domestic companies have released positive study results of their vaccine candidates for cervical cancer at the 2021 annual meeting held by the American Society of Clinical Oncology (ASCO).
In an abstract of their phase 2 clinical trials in Korea, Genexine and Cellid said their vaccines showed durable antitumor activity with an immune response in patients with human papillomavirus (HPV) types 16 or 18 positive recurrent cervical cancer.
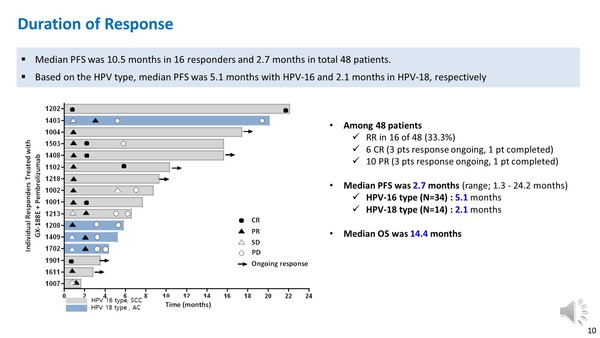
Genexine said its cervical cancer therapy, GX-188E vaccine, combined with Mercks’ Keytruda (ingredient: pembrolizumab), showed an enhanced response rate compared to Keytruda alone in phase 2 clinical trials.
The company assessed the data of 48 patients with recurrent or advanced cervical cancer in the study and confirmed that five patients had a complete response to the combo therapy. Ten patients also showed partial response, showing at least a 30 percent decrease of the target region.
The combination treatment showed a 31.3 percent overall response rate and 48 percent response rate in patients with PD-L1 and HPV 16-positive squamous cell carcinoma.
In the clinical trial, GX-188E and Keytruda treatment improved the median progression-free survival period to 4.1 months and overall survival up to 16.7 months, showing an enhanced result compared to monotherapy of Keytruda.
Seventeen of 52 patients enrolled in the study had mild adverse events of grade one or two, and only two participants showed grade three or four responses.
Genexine is conducting phase 2 studies to develop new anticancer drugs and plans to enroll up to 60 patients.
Cellid said that its cervical cancer vaccine BVAC-C showed a 21 percent overall response rate in the phase 2 study of twenty-one patients with HPV 16 or 18 positive recurrent cervical cancer who had experienced recurrence after one prior platinum-based combination chemotherapy.
The company said BVAC-C demonstrated a durable antitumor activity in HPV 16- or 18-positive cervical carcinoma patients who failed to respond to platinum-based chemotherapy.
The median progression-free survival in all patients was four months, and patients' immune responses after vaccination were correlated to clinical responses.
All evaluated patients showed inflammatory cytokine responses and potent E6/E7-specific T cell responses upon vaccinations, according to Cellid.

