NeoImmuneTech, an immuno-oncology and immunity-enhancing drug developer, has presented the study results of its NT-I7 (ingredient: efineptakin alfa) combined with Merck’s Keytruda (ingredient: pembrolizumab) for treating solid tumors.
According to the research presented at the 2021 meeting of the American Society of Clinical Oncology (ASCO), the combination of NT-I7 and Keytruda showed tolerance in patients with relapsed or refractory advanced solid tumors and increased the amount of T cells tumor microenvironment and peripheral blood.
The combo therapy of NT-I7 plus Keytruda has not caused severe adverse events while increasing T cells in the blood. It significantly increased the infiltration of T cells into the tumor microenvironment, the company said.
NT-I7 is the first-in-class long-acting recombinant human interleukin 7 (IL-7) that supports the proliferation and survival of CD4 and CD8 T-cells in humans and mice. The company’s previous studies demonstrated that NT-I7 could prevent lowered lymphocytes in blood and improve the survival of the orthotopic mouse model.
NeoImmuneTech carried out the study on 12 patients, evaluated the dose-limiting toxicity, and determined the maximum tolerated dose or recommended dose to continue in phase 2 clinical trials. The study also assessed the pharmacokinetics and preliminary antitumor activity of the combo therapy.
“One patient with metastatic mucosal melanoma who had not responded to the prior combination of nivolumab and ipilimumab showed a rapid partial response with a 46 percent tumor reduction,” NeoImmuneTech said through its poster presentation.
Adverse events related to the therapy occurred in 11 patients, and about one-third of them experienced grade one or two adverse events, but none of them suffered more severe responses. Patients who participated in the study showed adverse events, including injection site pain, chills, nausea, and pyrexia. However, no patients administered with the combo therapy underwent severe side effects, according to the company.
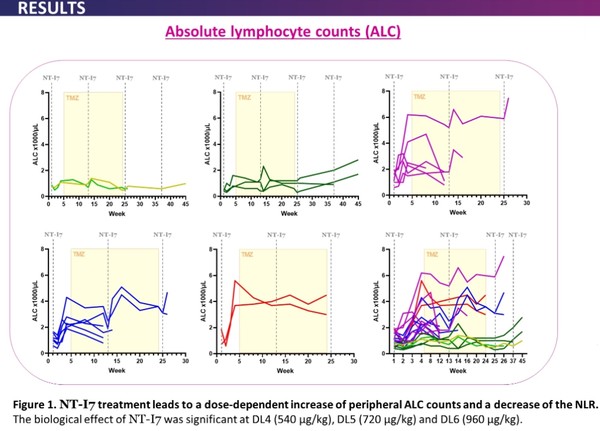
In another study released at the ASCO 2021, NT-I7 was well tolerated in patients with high-grade gliomas, a solid tumor in the brain.
NT-I7 treatment led to a dose-dependent increase of peripheral absolute lymphocyte count and a decrease of neutrophil-to-lymphocyte ratio (NLR) used as a biomarker to measure subclinical inflammation in humans.
The company said it plans to update the results of the ongoing studies and take the analysis into account in evaluating the effect of NT-I7.

