Sotorasib (brand name: Lumakras), the first KRAS mutation anticancer drug, has proved effective in non-small cell lung cancer (NSCLC) patients with various biomarkers, trial results showed.
The results of the sub-group analysis of the phase-2 CodeBreak100 trial were revealed at the metastatic NSCLC session during the meeting of the American Society of Clinical Oncology (ASCO 2021) on Saturday.
On May 28, the FDA granted the use of sotorasib for patients with KRAS G12C-mutated locally advanced or metastatic NSCLC who have received at least one prior systemic therapy. The drug became the first targeted therapy for KRAS mutation, which remained untreated for over 40 years.
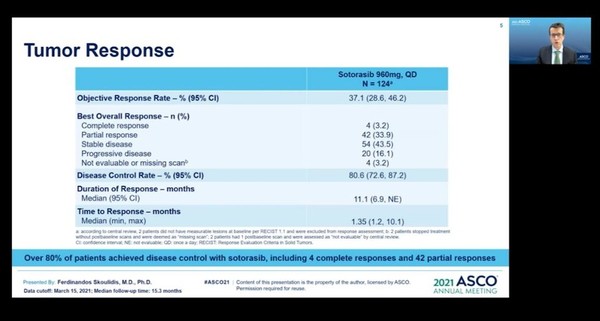
According to the presentation at ASCO 2021, 124 patients with KRAS G12C-mutated NSCLC patients whose disease progressed after prior treatment (chemotherapy and immunotherapy) took sotorasib 960 mg orally once a day in the study. Over 80 percent of the patients had experience receiving PD-1 or PD-L1 inhibitors in addition to platinum-based chemotherapy.
After the 15.3 months of the median follow-up, sotorasib-treated patients had a 37.1 percent objective response rate, including four complete responses (CR) and 42 partial responses (PR). The disease control rate (DCR) was 80.6 percent, and the duration of response (DoR) was 11.1 months.
The median progression-free survival (mPFS) was 6.8 months, and the median overall survival (mOS) was 12.5 months.
The safety profiles showed that treatment-related adverse events occurred in 88 patients (69.8 percent) out of 126. However, adverse reactions were mostly low grade, and 19.8 percent were grade 3 or higher, and 0.8 percent, grade 4 or higher.
Sotorasib demonstrated consistent effects in various sub-groups.
The response rate of patients previously treated with PD-1 or PD-L1 inhibitors (113) was 36.3 percent.
In addition, among those who had chemotherapy but not PD-1/PD-L1 inhibitors (11), the response rate was 45.5 percent. The response rate was as high as 69.2 percent in patients who had been treated with PD-1/PD-L1 inhibitors but not with chemotherapy (13).
In the analysis of subgroups with STK11 or KEAP11 or TP53 mutations known to have a poor diagnosis, the response rates were 40 percent in SKT11 mutated patients (35) and 39.3 percent in TP53 mutated patients (84).
Sotorasib’s efficacy was particularly pronounced in patients who had both wild-type KEAP1 and STK11 mutations. In this group, the ORR was 50 percent (11 out of 22), the mPFS, 11 months, and the mOS, 15.3 months.
The response rate in KEAP1 mutated patients was 20 percent, lower than the average of 37.1 percent, but this had limitations of small sample size, the research team said.
Professor Ferdinando's Skoulidis at the University of Texas MD Anderson Cancer Center, who presented the data at ASCO 2021, said sotorasib demonstrated continued clinical benefits without new safety concerns in previously treated patients with KRAS G12C mutated NSCLC.
“We confirmed the effect in molecularly defined subgroups including patients whose outcomes were poor after systemic therapy,” he said.
Sotorasib is also being tested in the phase-3 CodeBreak200 trial, which compares its effect with docetaxel in NSCLC patients with KRAS G12C mutation.

