SK Biopharmaceuticals said Wednesday that its partner, Angelini Pharma, began to market the Cenobamte under the brand name Ontozry in Germany early this month, marking its advance to the European market.
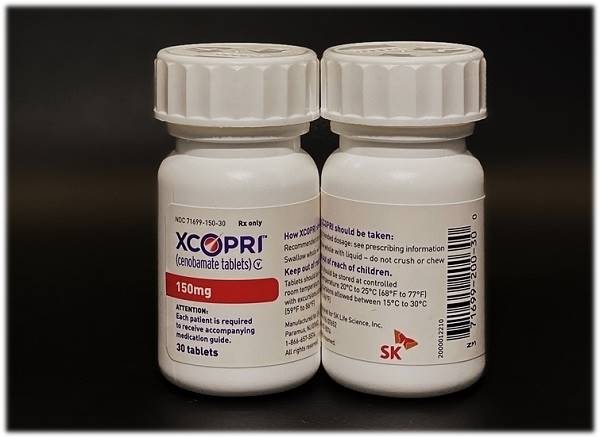
Cenobamate is an epilepsy therapy developed by SK Biopharmaceuticals. It was launched in the U.S., the world’s largest pharmaceutical market, with the name of Xcopri, last year, and made inroads in Europe, the second-largest market, in less than a year.
Germany has the largest pharmaceutical market in the EU, an outpost for entering Europe. According to the research firm Decision Resources Group, about 400,000 people with epilepsy are in the country. The World Health Organization notes that about 40 percent of epilepsy patients continue to have unexpected seizures despite taking multiple medications.
SK Biopharmaceuticals had accelerated marketing of Ontozry in Europe after receiving authorization from the European Commission in late March. The company is also to launch Ontozry in the U.K., as it received approval from the Medicines and Healthcare Products Regulatory Agency on June 4
The company expects to collect milestone payments and royalties linked to the sales of Ontozry from Angelini Pharma if the medicine’s sales expand to the rest of Europe.
SK Biopharmaceuticals initially signed an agreement with Swiss pharmaceutical company, Able Therapeutics, to commercialize Cenobamate in 41 European countries in 2019. The contract partner was changed to Angelini Pharma as the latter acquired Able Therapeutics in January.

