“If you can’t get a Tagrisso prescription, try a generic drug. The efficacy is almost the same. I hope you take the drug soon.”
“I also got to know a Tagrisso generic copy from Bangladesh. It is a little cheaper than the insurance-covered price.”
These comments about purchasing generic drugs of Tagrisso, a lung cancer treatment, were found in an online patient community comprised of 120,000 members.
The community had a posting that mentioned specific names of Tagrisso generics and which overseas internet site to go to. The article had over 20 comments asking how to buy a generic copy.
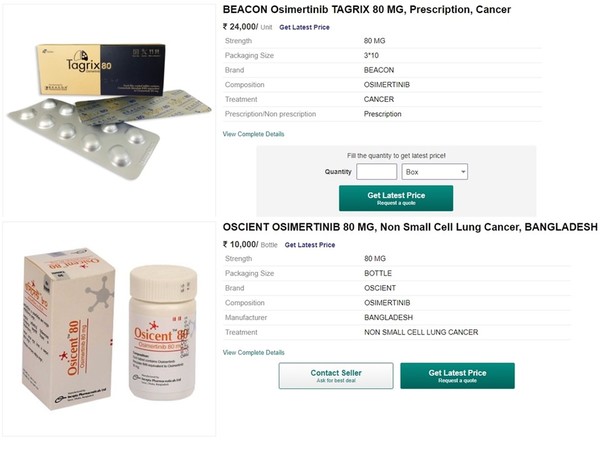
Korea Biomedical Review has checked an Indian e-commerce website with the information earned from the online patient community. There, Tagrix (ingredient: osimertinib) 80mg was priced at 24,000 rupees ($323) per box, and Osicent (osimertinib) 80mg, 10,000 rupees per box.
Tagrisso won local approval in 2016. It is prescribed to treat patients with non-small cell lung cancer (NSCLC) with EGFR exon 19 deletion or exon 21 substitution mutation after complete tumor resection. It is also used for the first-line treatment of locally advanced or metastatic NSCLC with exon 21 substitution mutation and patients with EGFR T790M mutation-positive, locally advanced, or metastatic NSCLC who have been previously treated with EGFR-TKI.
The Korean law bans purchasing drugs online. Tagrisso is an anticancer drug that requires a doctor’s prescription. Although cancer patients are aware of this, they risk the illegal purchase of Tagrisso generics because Tagrisso is too expensive.
Since Tagrisso won reimbursement for the second-line treatment of EGFR T790M mutated NSCLC patients in December 2017, it has still been used as the second-line therapy in Korea so far.
However, in other countries, including the U.S., Germany, Italy, Switzerland, the U.K., France, and Japan, Tagrisso is used as the first-line standard treatment.
Thus, Korean lung cancer patients who want to take Tagrisso in the first-line treatment have to pay several million won per month. Recently, lung cancer patients urged the government and AstraZeneca to expand reimbursement for Tagrisso.
Patients wanting Tagrisso in the first-line treatment have even gone further to buy Tagrisso generics overseas illegally.
The problem is that the online trading of drugs is not only illegal, but the effectiveness and safety of such generic copies are not guaranteed.
“A generic copy (purchased through an overseas website) is less safe and less effective than the original drug,” a member of the online patient community said.
“But I heard that people who took the generics said they were effective.”
The community member went on to say that an NSCLC patient with EGFR mutation could die without knowing Tagrisso was available just because the patient did not show T790M mutation.
Lee Geon-ju, leader of the Lung Cancer Patient Association, said he was completely unaware of some patients’ information sharing about the illegal online purchase of Tagrisso generics.
“I could understand how desperate patients can be, but wouldn’t it make their life too trivial to gamble with their lives,” he said. “Many people also try to make profits through such trading. That is like deceiving a patient whose life is at stake.”
Lee also noted that although people think that Korea has an excellent health insurance program, he could spot loopholes after becoming a lung cancer patient himself.
Physicians and health officials said they never heard of lung cancer patients trying to buy Tagrisso generics overseas. They warned against the act, saying patients could misuse or abuse the drugs and suffer adverse reactions.
Professor Lee Seung-hyun of pulmonology at Kyung Hee University Hospital said it was true that Tagrisso was effective for EGFR mutation-positive patients, particularly those with brain metastasis.
However, Tagrisso failed to win reimbursement despite the marketing permit for EGFR mutation-positive patients, he said.
“If a patient wishes to take the medication without insurance benefits, the patient has to pay over 4 million won per month even with the drug cost support from a patient support program,” Lee said.
Lee wondered how another pharmaceutical company made the generic drug of Tagrisso because the patent of Tagrisso has not expired. “The generic drug is illegal. I’m afraid its effect will be the same as that of the original drug,” he said.
Although he understood that lung cancer patients wanted to do everything they could, buying a generic drug from an international online site was a dangerous attempt, he said.
Generic medicines that copy the original drug require the regulator’s verification. The regulator checks whether the generic drug manufacturer used the verified material, complied with the dosage, and produced the product at a certified site. Then, it approves the generic copy.
However, it was not clear whether the generic drugs of Tagrisso being sold overseas underwent the regulator’s review.
One of the illegal generic drugs of Tagrisso, which was presumed to have been illegally imported into Korea, is packaged in a plastic bottle. In contrast, the original drug Tagrisso is individually packaged.
Lee said individual packaging is required for agents that absorb a lot of moisture.
If the pills are contained together in a plastic bottle, the moisture could affect the drug's efficacy, he said.

