Vivozon Healthcare said Thursday that it has received approval from the Institutional Review Boards (IRBs) of Korea’s four major hospitals to conduct phase 3 clinical trials of its non-opioid analgesic injection, Opiranserin (VVZ-149).
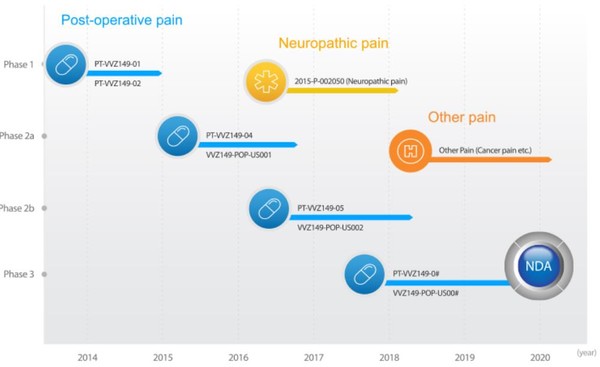
The company will conduct the phase 3 study in Seoul National University Hospital, Korea University Anam Hospital, Seoul National University Bundang Hospital, and Asan Medical Center with 300 patients who received surgery to remove rectum and colon.
Opiranserin is a non-opioid developmental candidate as an injectable agent to treat postoperative pain. IRB is a group designated to protect the rights, safety, and well-being of people involved in a clinical trial.
Vivozon aims to receive an indication for alleviating pain in patients who underwent laparoscopic colectomy, also called minimally invasive colectomy. The company believes VVZ-149 would replace opioid-based painkillers, as it could effectively reduce the patients’ pain by overcoming the adverse effects of non-steroidal anti-inflammatory drugs (NSAID).
The company acquired exclusive rights to provide Opiranserin in the domestic market in October 2020 and began conducting clinical trials in Korea.
“All four IRBs have approved the clinical trials and speed up the process and enroll the first patients,” Vivozon CEO Lee Doo-hyun said. “If the figure of SPID12 -- the sum of the difference in pain intensity over 12 hours -- meets the primary endpoint and end up with significantly higher results than the placebo group in this study, we will enter the next step to receive approval for laparoscopic and open surgery.”

