Ono Pharmaceutical’s Braftovi recently obtained local approval as the first targeted therapy to treat BRAF V600E mutant metastatic colorectal cancer (CRC).
However, industry watchers questioned why the drugmaker did not seek the permit for Mektovi, a drug used in combination with Braftovi to provide full treatment for such CRC patients.
On Aug. 19, the Ministry of Food and Drug Safety authorized Braftovi (encorafenib) as a combination therapy with cetuximab in adult patients with metastatic CRC who have been previously treated and whose BRAF V600E mutation was confirmed.
About 15 percent of CRC patients have BRAF V600E mutation in the early disease stage and 4.7 percent in the metastatic stage.
Patients with BRAF V600E-mutated metastatic CRC have a poor prognosis, and their survival is half that of metastatic patients without the mutation. There has been no authorized medicine that worked against BRAF gene-mutated CRC. So, patients have been desperately waiting for a new treatment.
Under such circumstances, Braftovi became the first targeted therapy in Korea to treat local metastatic CRC patients with BRAF V600E mutation.
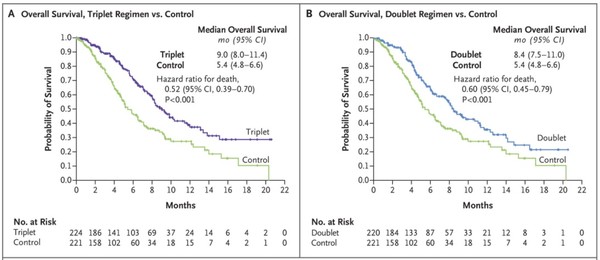
The permit for Braftovi was based on results from a phase 3 BEACON CRC study in patients with BRAF V600E mutation-positive advanced or recurrent CRC who have previously received treatment once or twice.
In the study, the Braftovi+cetuximab combo demonstrated a statistically significant extension of overall survival (OS) compared to irinotecan+cetuximab. In addition, the Braftovi+cetuximab combo lowered the risk of death by 40 percent. The median OS was 8.4 months in the Braftovi combo group vs. 5.4 months in the control group.
Also, the Braftovi plus cetuximab combo significantly improved the objective response rate (ORR). The ORR in the Braftovi combo group stood at 20 percent, statistically higher than 2 percent of the control group. The median progression-free survival (PFS) was 4.2 months in the Braftovi group vs. 1.5 months in the control group. This meant that Braftovi combo therapy lowered the risk of disease progression and death by 60 percent.
However, industry watchers noted that the trial did not intend to evaluate the combo of Braftovi and cetuximab.
The BEACON CRC trial’s main goal was to evaluate the three-drug combo Braftovi, Mektovi (binimetinib), and cetuximab compared with the irinotecan plus cetuximab combo. The study assessed the Braftovi plus cetuximab as well, but the evaluation was the secondary endpoint.
The trial results showed that the three-drug therapy had 26 percent ORR, nine months of median OS, and 4.3 months of median PFS. Its effect was most pronounced compared to the two-drug combo and the control group.
However, 58 percent of the three-drug therapy group, 50 percent of the two-drug group, and 61 percent of the control group experienced Grade 3 or more serious adverse reactions, indicating that adding Mektovi could increase side effects.
Still, researchers are testing the Braftovi+Mektovi+cetuximab in patients with BRAF V600E-mutated CRC who have no treatment history in a phase 2 ANCHOR CRC trial.
Ono Pharmaceutical, which owns the right to develop and commercialize Braftovi and Mektovi, said it did not plan to introduce Mektovi in Korea.
The move contrasts with the drugmaker’s obtaining of marketing approval for both the three-drug combo Braftovi+Mektovi+cetuximab and the two-drug combo Braftovi+cetuximab in Japan in November.
Ono Pharmaceutical obtained the right to develop and commercialize Braftovi and Mektovi in Japan and Korea from Array BioPharma Inc. in May 2017.

