The number of clinical trials to confirm the safety and efficacy of software medical devices is increasing steadily, government data showed.
The trend reflects the recognition of software medical devices as the key technology to meet a new demand in healthcare where physicians’ focus of patient care is shifting from treatment to prevention, observers said.
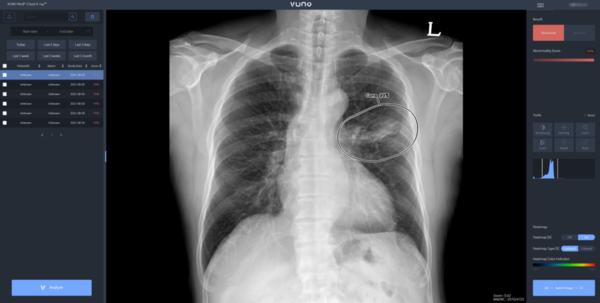
On Friday, the Ministry of Food and Drug Safety said it approved 21 trials of software medical devices in 2020.
The number of trials has been steadily rising from six in 2018 to 19 in 2019 and 21 in 2020.
The authorized studies tested various kinds of software medical devices. In 2018, there were only two kinds of such devices. However, the number grew to five in 2019, and seven in 2020.
By type, there are medical image detection assistance software, cancer diagnostic test software, medical image analysis device software, and disease diagnostic test software.
Among them, medical imaging diagnosis assistance software had the highest number of trials every year – three in 2018, six in 2019, and 11 in 2020.
For software medical device trials, researchers widely use retrospective methods using patient data such as medical and diagnostic records.
Out of the 46 authorized studies on software medical devices from 2018 to 2020, 42 were retrospective trials, and four, prospective.
Among the 14 products designated by the MFDS as innovative medical devices, nine are software. Three out of the nine device manufacturers received certification as “an innovative medical device software manufacturer.”
In Korea, developers of software medical devices include Lunit, VUNO, JLK, Coreline Soft, Medical IP, Medi Whale, Medical AI, Laon People, and Monitor Corporation.
Industry officials said the development and release of medical device software will become more active.
“The advanced AI medical imaging analysis technology provides quantified information for medical professionals’ experience-based interpretation, adding a reliable objective basis to the medical staff,” Medical IP CEP Park Sang-joon said. “This allows physicians to check the patient’s condition and treatment progress more accurately.”

