The Covid-19 pandemic limited in-person business activities, but digital transformation strategies could help yield even higher profits for local pharmaceutical and biotech companies, an industry official said.
James Choi, senior vice president and chief information and marketing officer at Samsung Biologics, spoke on the digital transformation of biologic drug production at the Global Bio Conference on Wednesday.
Choi pointed out that the pharmaceutical and biotech sector is relatively conservative in digitization compared to the automobile and electronics industries.
As product safety is important and regulations are strict in the pharma industry, local drugmakers tend to respond to changes slowly, and their digital maturity is low, he said.
He also said pharmaceutical companies had not made sufficient efforts to digitize their business. “There are not many efforts to achieve a return on investment (ROI) through digitization, and even large pharmaceutical companies still use old practices,” Choi said.
However, digitization could help secure high quality and data integrity in drug production, raise work efficiency, save costs by preventing information from being fragmented, and optimize the development process, according to Choi. Ultimately, he explained that digitization improves customer satisfaction and helps deliver a better drug more quickly to patients.
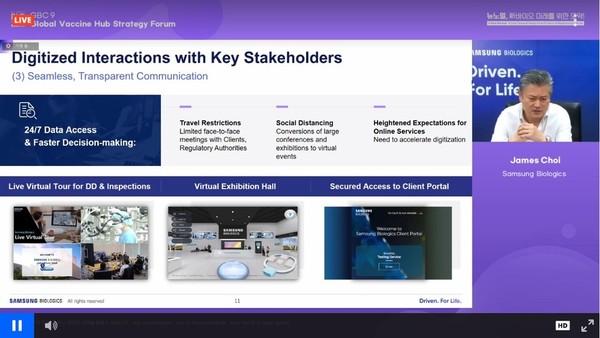
Samsung Biologics is actively using digital methods in communication with client companies and regulatory agencies.
Choi said the company introduced the “Live Virtual Tour (LVT)” technology in January 2020 to provide remote site inspections while maintaining high-definition and stable connections.
The company adopted the technology to overcome the difficulty of in-person inspection caused by the pandemic and strict social distancing last year.
“One of the most important businesses for Samsung Biologics is a client firm’s inspection on our company’s factory. This is because the client firm has to check whether our quality process and manufacturing capacity meet their requirements,” Choi said. “Inspections are important also for regulatory agencies, but Covid-19 made it difficult to do it.”
Recently, Choi realized that his meetings with potential clients were starting to shift to contactless ones, he said.
Also, Samsung Biologics has realized the “Virtual Person-in-Plant” so that client companies worldwide can virtually supervise drug development or production. Furthermore, the digital solution allows clients to check quality records and see what improvements have been made and what kind of work has been done in real-time.
Samsung Biologics also installed the Virtual Exhibition Hall.
The company’s digitization efforts enabled the FDA’s virtual inspection for a Covid-19 treatment, Choi said.
“We are probably the first company to receive FDA inspection using virtual tour technology,” he said. The virtual inspection was possible because the product was related to Covid-19 treatment, and we already had a digital transformation technology, in addition to the FDA’s active cooperation, Choi went on to say. “Our investment in the digital platform technology greatly helped.”
Choi said Samsung Biologics had a bold and aggressive roadmap for digitization to raise product quality, adding that prospects on IoT, big data, and blockchain technology were bright.
However, he cautioned against the reckless introduction of new technologies.
“What’s important is not going digital because it is a new technology but because it helps business operation,” he said. A company should secure specific superior competitiveness, which should help provide a better service for clients, he added.
Asked what Korea should prepare to become a global biotech hub in the pandemic era, Choi said the Korean regulator should actively introduce technologies that foreign regulatory agencies are reviewing for digital transformation.
The FDA adopts new technologies swiftly, not just for inspection but approval. Guidelines of the European Medicines Agency (EMA) or Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) often mention technologies such as data integrity and live virtual tour Choi said.
“It is wonderful to see what Korea has done in the New Normal era. Koreans are using digital technologies in daily lives well,” he said. “I highly anticipate that Korea can set a new model in the pharmaceutical and biotech industry.”

