Roche Korea said Tuesday that it would expand the company’s neuroscience unit by receiving approval for its two mental disorder treatments -- Evrysdi (ingredient: risdiplam) and Enspryng (satralizumab) -- in five years.
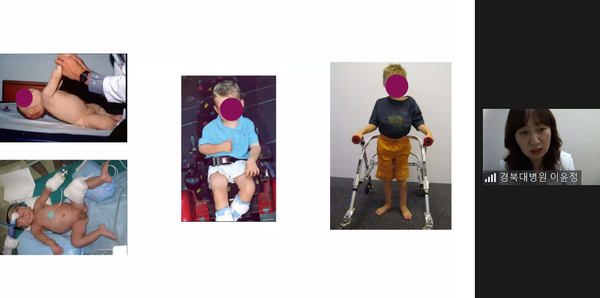
The company also stressed the importance of treating rare diseases, such as spinal muscular atrophy (SMA) and related neurological disorders, during an online conference to present its future plans for developing neurological disorder therapies.
Roche Korea established its neuroscience unit last November and has since spurred the development of treatments for rare disorders.
It now holds more than 15 pipelines concerning nerve disorders.
"We are preparing to receive approval for SRP-9001, an investigational drug candidate for Duchenne muscular dystrophy within five years, and expand our neuroscience business," Roche Korea's Neuroscience Squad Lead Jang Jung-hyun said. "We are also conducting a phase 3 clinical trial of gantenerumab for Alzheimer's disease to receive approval in five years."
Roche's Evrysdi is the first approved oral treatment for spinal muscular atrophy (SMA) patients. Enspryng is also the first at-home therapy for neuromyelitis optica spectrum disorder. SMA is a rare neuromuscular disease that causes patients to lose their motor nerves due to a deficiency in the survival motor neuron protein (SMN), essential for motor function.
Professor Lee Yun-jeong of the Department of Pediatrics at Kyungpook National University Hospital said, "SMA patients can develop symptoms at any point in life from infancy to adulthood, and those with type 3 muscular dystrophy that appear after 18 months of birth have an almost normal life expectancy, which makes why we need a treatment strategy to manage patients throughout all age groups continuously."
Lee pointed out that patients require early treatment even before they develop symptoms as they could suffer irreversible damage once they begin to show the symptoms. With the recent breakthroughs in developing medicine, SMA patients can enjoy life expectancy while maintaining their motor function and daily routine if they are diagnosed and properly treated at an early stage.
Evrysdi binds to the pre-mRNA of the SMN2 gene and increases and maintains the concentration of SMN by compensating for defects caused by a genetic mutation. In addition, the drug can cross the blood-brain barrier and distributes SMN protein throughout the body, including the central nervous system.
Roche presented new two-year data for the two major studies of SUNFISH and FIREFISH evaluating efficacy and safety of Evrysdi at the 2021 annual meeting of the American Academy of Neurology.
In the second part of the FIREFISH study, Evrysdi demonstrated efficacy and safety in 41 SMA patients with infantile-onset from 2.2 to 6.9 months of age. The study's primary endpoint was to see whether the babies could sit without assistance for at least five seconds.
After 24 months of treatment with Evrysdi, 61 percent of the patients could sit without help for at least five seconds, and 95 percent could swallow.
The company also confirmed the safety and efficacy of Evrysdi in the second part of the SUNFISH study conducted with 180 types 2 and type 3 SMA patients aged between two to 25 years.
The primary endpoint was the changes in 32 items of the motor function measure (MFM-32) compared to the baseline after a year. The study results showed that the patient maintained or improved motor or showed continuous improvement throughout two-year-long therapy.
"As the first and only oral treatment, patients can take Evrysdi once a day at home and expect continuous therapeutic efficacy," Roche Korea Medical Director Lee Seung-hoon said.
Neuroscience Squad Lead Jang also vowed that Roche Korea would continue its research and development to improve unmet medical needs and access to rare diseases treatments.

